Epidemiological Profile of Urinary and Intestinal Schistosomiasis in the Kingdom of Saudi Arabia: A Seven-Year Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Period
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Incidences and Distribution of Schistosoma Cases in KSA 2014–2020
3.2. Clinical Types of Schistosomiasis and Their Distribution among KSA Regions (2014–2020)
3.3. Gender Disparities in Schistosomiasis Distribution across Regions in KSA (2014–2020)
3.4. Nationality-Based Distribution of Schistosomiasis across Regions in KSA (2014–2020)
3.5. Age-Based Distribution of Schistosomiasis across Regions in KSA (2014–2020)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, H.S. Schistosomiasis (Bilharzia): Background, Pathophysiology, Etiology. 2023. Available online: https://emedicine.medscape.com/article/228392-overview?form=fpf (accessed on 30 March 2023).
- He, P.; Song, L.G.; Xie, H.; Liang, J.Y.; Yuan, D.Y.; Wu, Z.D.; Lv, Z.Y. Nucleic acid detection in the diagnosis and prevention of schistosomiasis. Infect. Dis. Poverty 2016, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Dangerfield-Cha, M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008, 4, 65–79. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, W.H.O. Inter-Country Meeting on Strategies to Eliminate Schistosomiasis from the Eastern Mediterranean Region; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Barakat, R.M.; Morshedy, H.E.; Farghaly, A.G. Human Schistosomiasis in the Middle East and North Africa Region; Springer: Vienna, Austria, 2014. [Google Scholar]
- World Health Organization, W.H.O. The Social Context of Schistosomiasis and Its Control: An Introduction and Annotated Bibliography; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Hotez, P.J.; Savioli, L.; Fenwick, A. Neglected tropical diseases of the Middle East and North Africa: Review of their prevalence, distribution, and opportunities for control. PLoS Negl. Trop. Dis. 2012, 6, e1475. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, M.J.; de Vlas, S.J.; Brooker, S.; Looman, C.W.; Nagelkerke, N.J.; Habbema, J.D.; Engels, D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003, 86, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Clerinx, J.; Van Gompel, A. Schistosomiasis in travellers and migrants. Travel Med. Infect. Dis. 2011, 9, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, K.G.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the Diagnosis of Human Schistosomiasis. Clin. Microbiol. Rev. 2015, 28, 939–967. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.J.; de Dood, C.J.; Lewis, M.; Deelder, A.M.; van Lieshout, L.; Tanke, H.J.; van Rooyen, L.H.; Corstjens, P.L. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. Parasitol. 2013, 135, 274–282. [Google Scholar] [CrossRef]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef]
- Célestin Kyambikwa, B. Epidemiology and Control of Schistosomiasis. In New Horizons for Schistosomiasis Research; Tonay, I., Ed.; IntechOpen: Rijeka, Yugoslavia, 2022; chapter. 2. [Google Scholar]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Arfaa, F. Studies on schistosomiasis in the Yemen Arab Republic. Am. J. Trop. Med. Hyg. 1972, 21, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Arfaa, F. Studies on schistosomiasis in Saudi Arabia. Am. J. Trop. Med. Hyg. 1976, 25, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.R.; Cannon, J.M.; Al Juburi, A.Z.; Cockett, A.T. Schistosomiasis in Saudi Arabia, Egypt, and Iraq. Urology 1998, 51, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Hatch, W.K. Bilharzia Haematobia. Br. Med. J. 1878, 2, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Sebai, Z.A. Schistosomiasis in Saudi Arabia. Ann. Saudi Med. 1988, 8, 169–174. [Google Scholar] [CrossRef]
- Lotfy, W.; Alsaqabi, S. Human schistosomiasis in the Kingdom of Saudi Arabia: A review. J. Med. Res. Inst. 2010, 31, 1–6. [Google Scholar]
- Arfaa, F.; Mahboubi, E.; al Jeffri, M.; Selim, A.; Russell, G. The potential role of various species of intermediate hosts of Schistosoma haematobium in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 216–218. [Google Scholar] [CrossRef]
- Bin Dajem, S.M. Freshwater snails in Asser region, Saudi Arabia with special refernce to the zoonotic trematode. J. Egypt Soc. Parasitol. 2009, 39, 551–557. [Google Scholar]
- Ghandour, A.M.; Zahid, N.Z.; Banaja, A.A. Epidemiological study on the transmission of schistosomiasis in Saudi Arabia (western region). Ann. Trop. Med. Parasitol. 1999, 93, 193–195. [Google Scholar] [CrossRef]
- el Gayar, S.B.; Baknina, M.H.; el Kady, N.M.; Abdel Hafez, M.A. First report of schistosomiasis japonica in Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 169. [Google Scholar] [CrossRef]
- al-Madani, A.A.; Mahfouz, A.A. Prevalence of intestinal parasitic infections among Asian female house keepers in Abha District, Saudi Arabia. Southeast Asian J. Trop. Med. Public Health 1995, 26, 135–137. [Google Scholar] [PubMed]
- Kalantan, K.A.; Al-Faris, E.A.; Al-Taweel, A.A. Pattern of intestinal parasitic infection among food handlers in riyadh, saudi arabia. J. Fam. Community Med. 2001, 8, 67–72. [Google Scholar] [CrossRef]
- Abahussain, N.A.; Abahussain, N. Prevalence of intestinal parasites among expatriate workers in Al-Khobar, Saudi Arabia. Middle E J. Fam. Med. 2005, 3, 17–21. [Google Scholar]
- Wakid, M.; Azhar, E.; Zafar, T.A. Intestinal Parasitic Infection among Food Handlers in the Holy City of Makkah During Hajj Season: 1428 Hegria (2007 G). J. King Abdulaziz Univ.-Med. Sci. 2009, 16, 39–52. [Google Scholar] [CrossRef]
- Mohammad, K.A.; Koshak, E.A. A prospective study on parasites among expatriate workers in Al-Baha from 2009–2011, Saudi Arabia. J. Egypt Soc. Parasitol. 2011, 41, 423–432. [Google Scholar] [PubMed]
- Ochsenwald, W.L.P.; Harry, S.J.B.; Teitelbaum, J. Geography & Travel Saudi Arabia. 2023. Available online: https://www.britannica.com/place/Saudi-Arabia (accessed on 30 March 2023).
- General Authority for Statistics 2023. Available online: https://www.stats.gov.sa/en (accessed on 15 January 2023).
- Saudi Arabia Climate: Average Weather, Temperature, Rain—Climates to Travel. Available online: https://www.climatestotravel.com/climate/saudi-arabia (accessed on 24 August 2023).
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuem Tchuente, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Russell, R.J.; Charters, A.D. A review of schistosomiasis in immigrants in Western Australia, demonstrating the unusual longevity of Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Giboda, M.; Bergquist, N.R. Post-transmission schistosomiasis: A new agenda. Acta Trop. 2000, 77, 3–7. [Google Scholar] [CrossRef]
- Fulford, A.J.; Butterworth, A.E.; Ouma, J.H.; Sturrock, R.F. A statistical approach to schistosome population dynamics and estimation of the life-span of Schistosoma mansoni in man. Parasitology 1995, 110 Pt 3, 307–316. [Google Scholar] [CrossRef]
- Vermund, S.H.; Bradley, D.J.; Ruiz-Tiben, E. Survival of Schistosoma mansoni in the human host: Estimates from a community-based prospective study in Puerto Rico. Am. J. Trop. Med. Hyg. 1983, 32, 1040–1048. [Google Scholar] [CrossRef]
- Mohammad, K.A. Prevalence of schistosomiasis in Al-Baha Province, Saudi Arabia in years 2012 and 2013 (prospective and comparative study). J. Egypt Soc. Parasitol. 2014, 44, 397–404. [Google Scholar] [CrossRef] [PubMed]
| Year | Total Population | Number of Examined | Positive n (%) | Negative n (%) | Incidence/100,000 Population | p Value | OR(95%CI) |
|---|---|---|---|---|---|---|---|
| 2014 | 28,309,273 | 787,699 | 117 (0.0148) | 787,582 (98.6) | 0.413 | <0.001 | 1.41 (0.98–2.03) ns |
| 2015 | 29,816,382 | 781,366 | 159 (0.0203) | 781,207 (97.97) | 0.533 | 1.93 (1.36–2.74) *** | |
| 2016 | 30,954,198 | 726,216 | 119 (0.0164) | 726,097 (98.36) | 0.384 | 1.56 (1.08–2.23) ** | |
| 2017 | 30,977,355 | 593,011 | 103 (0.0174) | 592,908 (98.26) | 0.332 | 1.65 (1.14–2.38) ** | |
| 2018 | 30,196,281 | 587,608 | 96 (0.0163) | 587,512 (98.37) | 0.318 | 1.55 (1.07–2.25) * | |
| 2019 | 30,063,799 | 525,301 | 47 (0.0089) | 525,254 (99.11) | 0.156 | 0.85 (0.56–1.29) ns | |
| 2020 | 31,552,510 | 370,280 | 39 (0.0105) | 370,241 (98.95) | 0.123 | Reference | |
| Total | - | 4,371,481 | 680 (0.0155) | 4,370,801 (98.45) | 2.155 | - |
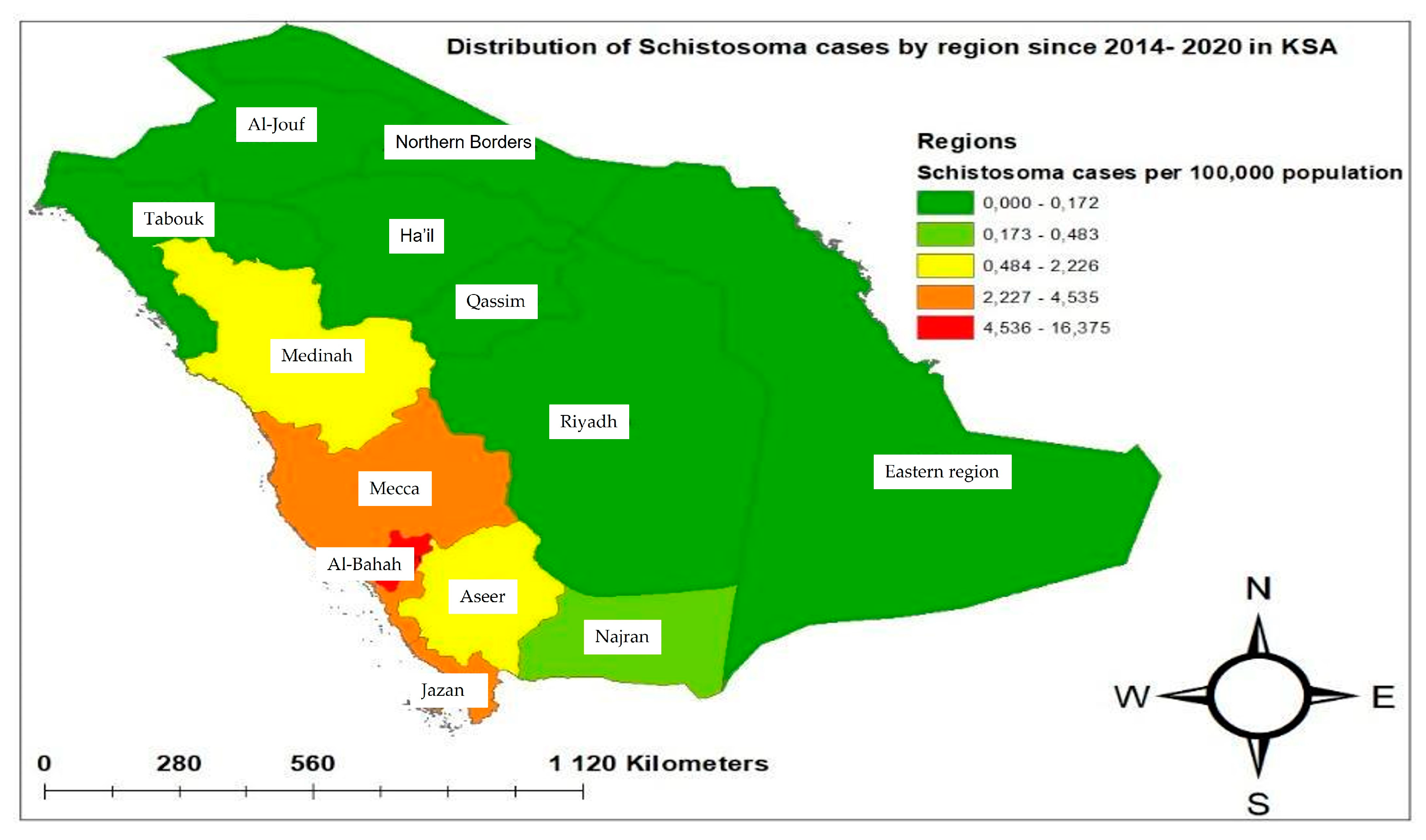
| Regions | Population | Number of Examined | Positive Cases | Cumulative Incidence per 100,000 | OR(95%CI) |
|---|---|---|---|---|---|
| Mecca | 9,261,257 | 1,026,438 | 420 | 4.54 | 5.57 (2.49–12.47) *** |
| Medinah | 2,291,092 | 682,426 | 51 | 2.22 | 1.02 (0.44–5.36) ns |
| Aseer | 2,354,320 | 278,936 | 47 | 1.59 | 2.29 (0.98–1.93) * |
| Jazan | 1,670,569 | 554,056 | 70 | 4.19 | 1.72 (0.75–3.96) ns |
| Al-Bahah | 506,866 | 454,227 | 83 | 16.4 | 2.49 (1.09–5.69) * |
| Eastern region | 3,485,383 | 81,673 | 6 | 0.17 | 0.09 (0.02–0.39) *** |
| Najran | 621,040 | 419,683 | 3 | 0.49 | Reference |
| Total | 26,550,777 | 4,371,481 | 680 | 2.56 | - |
| Regions | Type of Schistosomiasis | Total n (%, Out of All Positive Cases) | p Value | |
|---|---|---|---|---|
| Urinary n (%) | Intestinal n (%) | |||
| Mecca | 22 (3.2) | 398 (58.5) | 420 (61.7) | <0.001 |
| Medinah | 11 (1.6) | 40 (5.9) | 51 (7.5) | |
| Aseer | 27 (4.0) | 20 (3.0) | 47 (7.0) | |
| Jazan | 69 (10.1) | 1 (0.15) | 70 (10.3) | |
| Najran | 2 (0.29) | 1 (0.15) | 3 (0.4) | |
| Al-Bahah | 3 (0.44) | 80 (11.8) | 83 (12.2) | |
| Eastern region | 5 (0.74) | 1 (0.15) | 6 (0.9) | |
| Total | 139 (20.4) | 541 (79.6) | 680 (100) | |
| Regions | Gender | Total n (%, Out of All Positive Cases) | p Value | |
|---|---|---|---|---|
| Male n (%) | Female n (%) | |||
| Mecca | 371 (54.5) | 49 (7.2) | 420 (61.7) | <0.001 |
| Medinah | 44 (6.5) | 7 (1.0) | 51 (7.5) | |
| Aseer | 42 (6.2) | 5 (0.7) | 47 (7.0) | |
| Jazan | 52 (7.6) | 18 (2.6) | 70 (10.3) | |
| Najran | 3 (0.44) | 0 | 3 (0.4) | |
| Al-Bahah | 68 (10) | 15 (2.2) | 83 (12.2) | |
| Eastern region | 4 (0.59) | 2 (0.3) | 6 (0.9) | |
| Total | 584 (85.9) | 96 (14.1) | 680 (100) | |
| Regions | Nationality | Total n (%, Out of All Positive Cases) | p Value | |
|---|---|---|---|---|
| Saudi n (%) | Expatriates n (%) | |||
| Mecca | 87 (12.7) | 333 (49) | 420 (61.7) | <0.001 |
| Medinah | 0 | 51 (7.5) | 51 (7.5) | |
| Aseer | 16 (2.3) | 31 (4.6) | 47 (7.0) | |
| Jazan | 15 (2.2) | 55 (8.1) | 70 (10.3) | |
| Najran | 0 | 3 (0.44) | 3 (0.4) | |
| Al-Bahah | 78 (11.5) | 5 (0.7) | 83 (12.2) | |
| Eastern region | 4 (0.6) | 2 (0.3) | 6 (0.9) | |
| Total | 200 (29.4) | 480 (70.6) | 680 (100) | |
| Regions | Age Groups | Total n (%, Out of All Positive Cases) | p Value | |||
|---|---|---|---|---|---|---|
| <5 Years n (%) | 5–14 Years n (%) | 15–39 Years n (%) | >40 Years n (%) | |||
| Mecca | 0 | 44 (6.5) | 335 (49.2) | 41 (0.6) | 420 (61.7) | <0.001 |
| Medinah | 0 | 0 | 44 (6.6) | 7 (1.0) | 51 (7.5) | |
| Aseer | 1 (0.15) | 19 (2.8) | 26 (3.8) | 1 (0.15) | 47 (7.0) | |
| Jazan | 0 | 14 (2.1) | 53 (7.8) | 3 (0.44) | 70 (10.3) | |
| Najran | 0 | 0 | 3 (0.4) | 0 | 3 (0.4) | |
| Al-Bahah | 0 | 28 (4.1) | 46 (6.8) | 9 (1.3) | 83 (12.2) | |
| Eastern region | 0 | 0 | 6 (0.9) | 0 | 6 (0.9) | |
| Total | 1 (0.1) | 105 (15.4) | 513 (75.5) | 61 (9.0) | 680 (100) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zrieq, R.; Alzain, M.A.; Ali, R.M.; Alazzeh, A.Y.; Tirawi, A.O.; Attili, R.; Acar, T.; Haouas, N. Epidemiological Profile of Urinary and Intestinal Schistosomiasis in the Kingdom of Saudi Arabia: A Seven-Year Retrospective Study. Trop. Med. Infect. Dis. 2024, 9, 11. https://doi.org/10.3390/tropicalmed9010011
Zrieq R, Alzain MA, Ali RM, Alazzeh AY, Tirawi AO, Attili R, Acar T, Haouas N. Epidemiological Profile of Urinary and Intestinal Schistosomiasis in the Kingdom of Saudi Arabia: A Seven-Year Retrospective Study. Tropical Medicine and Infectious Disease. 2024; 9(1):11. https://doi.org/10.3390/tropicalmed9010011
Chicago/Turabian StyleZrieq, Rafat, Mohamed Ali Alzain, Reem M. Ali, Awfa Y. Alazzeh, Anas O. Tirawi, Rozan Attili, Tolgahan Acar, and Najoua Haouas. 2024. "Epidemiological Profile of Urinary and Intestinal Schistosomiasis in the Kingdom of Saudi Arabia: A Seven-Year Retrospective Study" Tropical Medicine and Infectious Disease 9, no. 1: 11. https://doi.org/10.3390/tropicalmed9010011
APA StyleZrieq, R., Alzain, M. A., Ali, R. M., Alazzeh, A. Y., Tirawi, A. O., Attili, R., Acar, T., & Haouas, N. (2024). Epidemiological Profile of Urinary and Intestinal Schistosomiasis in the Kingdom of Saudi Arabia: A Seven-Year Retrospective Study. Tropical Medicine and Infectious Disease, 9(1), 11. https://doi.org/10.3390/tropicalmed9010011






