The Yield of Active Tuberculosis Disease and Latent Tuberculosis Infection in Tuberculosis Household Contacts Investigated Using Chest X-ray in Yogyakarta Province, Indonesia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
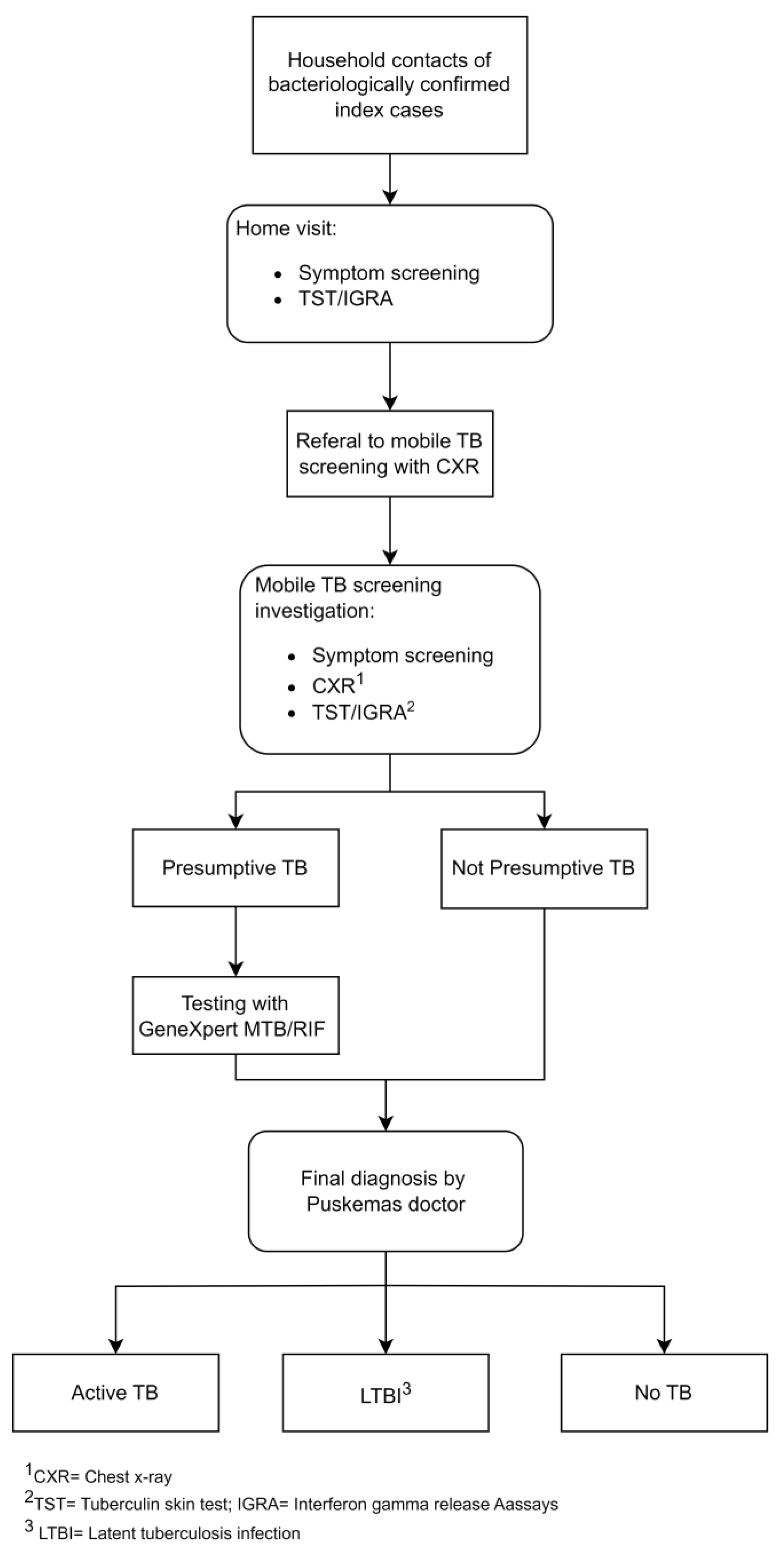
2.4. Study Procedures and Data Collection
2.5. Variables
3. Results
3.1. Active TB Cases Cascade among Household Contacts
3.2. Latent TB Infection Cascade among Household Contact
3.3. Factors Associated with Active TB Disease among Household Contacts
3.4. Factors Associated with Latent TB Infection among Household Contacts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Nelson, K.N.; Gandhi, N.R.; Mathema, B.; Lopman, B.A.; Brust, J.C.M.; Auld, S.C.; Ismail, N.; Omar, S.V.; Brown, T.S.; Allana, S.; et al. Modeling Missing Cases and Transmission Links in Networks of Extensively Drug-Resistant Tuberculosis in KwaZulu-Natal, South Africa. Am. J. Epidemiol. 2020, 189, 735–745. [Google Scholar] [CrossRef]
- Direktorat Jenderal Pencegahan dan Pengendalian Penyakit Kementerian Kesehatan Republik Indonesia Petunjuk Teknis Investigasi Kontak Pasien TBC Bagi Petugas Kesehatan Dan Kader; Kementerian Kesehatan Republic Indonesia: Jakarta, Indonesia, 2019; pp. 35–36.
- Ministry of Health of the Republic of Indonesia. TB Indonesia. © 2023 TBC Indonesia. Available online: https://Tbindonesia.or.Id/ (accessed on 27 September 2023).
- WHO Consolidated Guidelines on Tuberculosis. Module 2: Systematic Screening for Tuberculosis Disease; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Hossain, A.D.; Jarolimova, J.; Elnaiem, A.; Huang, C.X.; Richterman, A.; Ivers, L.C. Effectiveness of Contact Tracing in the Control of Infectious Diseases: A Systematic Review. Lancet Public Health 2022, 7, e259–e273. [Google Scholar] [CrossRef]
- Badan Pusat Statistik Provinsi D.I Yogyakarta. Analisis Profil Penduduk Analisis Tematik Kependudukan: Peran Lintas Generasi Dalam Pembangunan D.I.Yogyakarta; Badan Pusat Statistik Provinsi D.I Yogyakarta: Kasihan, Indonesia, 2022. [Google Scholar]
- World Health Organization. WHO Operational Handbook on Tuberculosis, Module 1: Prevention: Tuberculosis Preventive Treatment; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789240002906. [Google Scholar]
- Tham, K.W.; Abdul Ghani, R.; Cua, S.C.; Deerochanawong, C.; Fojas, M.; Hocking, S.; Lee, J.; Nam, T.Q.; Pathan, F.; Saboo, B.; et al. Obesity in South and Southeast Asia—A New Consensus on Care and Management. Obes. Rev. 2023, 24, e13520. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)-A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Fox, G.J.; Barry, S.E.; Britton, W.J.; Marks, G.B. Contact Investigation for Tuberculosis: A Systematic Review and Meta-Analysis. Eur. Respir. J. 2013, 41, 140–156. [Google Scholar] [CrossRef]
- Gyawali, N.; Gurung, R.; Poudyal, N.; Amatya, R.; Niraula, S.R.; Jha, P.; Bhattacharya, S.K. Prevalence of tuberculosis in household contacts of sputum smears positive cases and associated demographic risk factors. Nepal. Med. Coll. J. 2012, 14, 303–307. [Google Scholar]
- Mac, T.H.; Phan, T.H.; Van Nguyen, V.; Dong, T.T.T.; Van Le, H.; Nguyen, Q.D.; Nguyen, T.D.; Codlin, A.J.; Mai, T.D.T.; Forse, R.J.; et al. Optimizing Active Tuberculosis Case Finding: Evaluating the Impact of Community Referral for Chest X-ray Screening and Xpert Testing on Case Notifications in Two Cities in Viet Nam. Trop. Med. Infect. Dis. 2020, 5, 181. [Google Scholar] [CrossRef]
- Gupta, M.; Saibannavar, A.; Kumar, V. Household Symptomatic Contact Screening of Newly Diagnosed Sputum Smears Positive Tuberculosis Patients—An Effective Case Detection Tool. Lung India 2016, 33, 159. [Google Scholar] [CrossRef]
- Ghanaiee, R.M.; Karimi, A.; Hoseini-Alfatemi, S.M.; Seddon, J.A.; Nasehi, M.; Tabarsi, P.; Fahimzad, S.A.; Armin, S.; Akbarizadeh, J.; Rahimarbabi, E.; et al. Household Contact Investigation for the Detection of Active Tuberculosis and Latent Tuberculosis: A Comprehensive Evaluation in Two High-Burden Provinces in Iran. New Microbes New Infect. 2022, 45, 100958. [Google Scholar] [CrossRef]
- Iqbal, R.; Munir, K.; Arif, A.; Rao, M.H.; Mirbahar, A.; Akhtar, T.; Firdous, R.; Asim, M. Screening for Tuberculosis among Household Contacts of Index Patients. Pak. J. Med. Res. 2013, 52, 96–101. [Google Scholar]
- Nair, D.; Rajshekhar, N.; Klinton, J.S.; Watson, B.; Velayutham, B.; Tripathy, J.P.; Jawahar, M.S.; Swaminathan, S. Household Contact Screening and Yield of Tuberculosis Cases-a Clinic Based Study in Chennai, South India. PLoS ONE 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Beyanga, M.; Kidenya, B.R.; Gerwing-Adima, L.; Ochodo, E.; Mshana, S.E.; Kasang, C. Investigation of Household Contacts of Pulmonary Tuberculosis Patients Increases Case Detection in Mwanza City, Tanzania. BMC Infect Dis 2018, 18, 110. [Google Scholar] [CrossRef]
- Tuberculosis Coalition for Technical Assistance. International Standards for Tuberculosis Care, 3rd ed.; Tuberculosis Coalition for Technical Assistance: The Hague, The Netherlands, 2014. [Google Scholar]
- Shewade, H.D.; Gupta, V.; Ghule, V.H.; Nayak, S.; Satyanarayana, S.; Dayal, R.; Mohanty, S.; Singh, S.; Biswas, M.; Kumar Reddy, K.; et al. Impact of Advocacy, Communication, Social Mobilization and Active Case Finding on TB Notification in Jharkhand, India. J. Epidemiol. Glob. Health 2019, 9, 233–242. [Google Scholar] [CrossRef]
- Mhimbira, F.A.; Cuevas, L.E.; Dacombe, R.; Mkopi, A.; Sinclair, D. Interventions to Increase Tuberculosis Case Detection at Primary Healthcare or Community-Level Services. Cochrane Database Syst. Rev. 2017, 2017, 1858. [Google Scholar] [CrossRef]
- Vo, L.N.Q.; Forse, R.J.; Codlin, A.J.; Vu, T.N.; Le, G.T.; Do, G.C.; Van Truong, V.; Dang, H.M.; Nguyen, L.H.; Nguyen, H.B.; et al. A Comparative Impact Evaluation of Two Human Resource Models for Community-Based Active Tuberculosis Case Finding in Ho Chi Minh City, Viet Nam. BMC Public Health 2020, 20, 934. [Google Scholar] [CrossRef]
- Velleca, M.; Malekinejad, M.; Miller, C.; Abascal Miguel, L.; Reeves, H.; Hopewell, P.; Fair, E. The Yield of Tuberculosis Contact Investigation in Low- and Middle-Income Settings: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2021, 21, 1011. [Google Scholar] [CrossRef]
- Krishnamoorthy, Y.; Ezhumalai, K.; Murali, S.; Rajaa, S.; Jose, M.; Sathishkumar, A.; Soundappan, G.; Horsburgh, C.; Hochberg, N.; Johnson, W.E.; et al. Prevalence and Risk Factors Associated with Latent Tuberculosis Infection among Household Contacts of Smear Positive Pulmonary Tuberculosis Patients in South India. Trop. Med. Int. Health 2021, 26, 1645–1651. [Google Scholar] [CrossRef]
- Tornee, S.; Kaewkungwal, J.; Fungladda, W.; Silachamroon, U.; Akarasewi, P.; Sunakorn, P. Risk Factors for Tuberculosis Infection among Household Contacts in Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2004, 35, 375–383. [Google Scholar]
- Karbito, K.; Susanto, H.; Adi, M.S.; Sulistiyani, S.; Handayani, O.W.K.; Sofro, M.A.U. Latent Tuberculosis Infection in Family Members in Household Contact with Active Tuberculosis Patients in Semarang City, Central Java, Indonesia. J. Public Health Afr. 2022, 13, 2157. [Google Scholar] [CrossRef]
- Frascella, B.; Richards, A.S.; Sossen, B.; Emery, J.C.; Odone, A.; Law, I.; Onozaki, I.; Esmail, H.; Houben, R.M.G.J. Subclinical Tuberculosis Disease—A Review and Analysis of Prevalence Surveys to Inform Definitions, Burden, Associations, and Screening Methodology. Clin. Infect. Dis. 2021, 73, e830–e841. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhao, F.; Xia, Y.Y.; Yu, Y.L.; Shen, X.; Lu, W.; Wang, X.M.; Xing, J.; Ye, J.J.; Li, J.W.; et al. Prevalence and Risk Factors of Active Pulmonary Tuberculosis among Elderly People in China: A Population Based Cross-Sectional Study. Infect. Dis. Poverty 2019, 8, 26–35. [Google Scholar] [CrossRef]
- Odera, S.; Mureithi, M.; Aballa, A.; Onyango, N.; Anzala, O.; Oyugi, J. Latent Tuberculosis among Household Contacts of Pulmonary Tuberculosis Cases in Nairobi, Kenya. Pan Afr. Med. J. 2020, 37, 1–14. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, S.H.; Kim, Y.E.; Cho, Y.J.; Jeong, Y.Y.; Kim, H.C.; Lee, J.D.; Kim, J.R.; Hwang, Y.S.; Kim, H.J.; et al. Risk Factors for Latent Tuberculosis Infection in Close Contacts of Active Tuberculosis Patients in South Korea: A Prospective Cohort Study. BMC Infect. Dis. 2014, 14, 566. [Google Scholar] [CrossRef]
- Pareek, M.; Watson, J.P.; Ormerod, L.P.; Kon, O.M.; Woltmann, G.; White, P.J.; Abubakar, I.; Lalvani, A. Screening of Immigrants in the UK for Imported Latent Tuberculosis: A Multicentre Cohort Study and Cost-Effectiveness Analysis. Lancet Infect. Dis. 2011, 11, 435–444. [Google Scholar] [CrossRef]
- Aabye, M.G.; Ravn, P.; PrayGod, G.; Jeremiah, K.; Mugomela, A.; Jepsen, M.; Faurholt, D.; Range, N.; Friis, H.; Changalucha, J.; et al. The Impact of HIV Infection and CD4 Cell Count on the Performance of an Interferon Gamma Release Assay in Patients with Pulmonary Tuberculosis. PLoS ONE 2009, 4, e4220. [Google Scholar] [CrossRef]
- Getahun, H.; Matteelli, A.; Abubakar, I.; Aziz, M.A.; Baddeley, A.; Barreira, D.; Den Boon, S.; Borroto Gutierrez, S.M.; Bruchfeld, J.; Burhan, E.; et al. Management of Latent Mycobacterium tuberculosis Infection: WHO Guidelines for Low Tuberculosis Burden Countries. Eur. Respir. J. 2015, 46, 1563–1576. [Google Scholar] [CrossRef]
- Kementerian Kesehatan Republik Indonesia. Petunjuk Teknis Penanganan Infeksi Laten Tuberkulosis (ILTB); Kementerian Kesehatan Republik Indonesia: Jakarta, Indonesia, 2020; ISBN 9786024169572. [Google Scholar]
| Variable | Symptom and CXR Screening + Bacteriological Testing N (%) | Symptom Screening Only + Bacteriological Testing N (%) |
|---|---|---|
| HHC symptoms screened | 2857 (100%) | 2857 (100%) |
| HHC CXR screened | 2368 (82.9%) | - |
| HHC presumptive TB | 455 (15.9%) | 216 (7.6%) |
| HHC diagnosed TB | 68 (2.4%) | 5 (0.2%) |
| HHC bacteriologically confirmed | 13 (0.5%) | 5 (0.2%) |
| Variable | Total N = 2857 (%) |
|---|---|
| Household contacts eligible for LTBI screening | 2621 (92.0%) |
| Household contacts tested for LTBI | 2371 (90.5%) |
| Tested with IGRA | 359 (15.1%) |
| Tested with TST | 2012 (84.9%) |
| Household contacts positive for LTBI | 1083 (45.7%) |
| Positive IGRA result | 124 (5.2%) |
| Positive TST result | 959 (40.4%) |
| Summary Statistics | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No TB Disease N = 2789 | Active TB Disease N = 68 1 | Total N = 2857 | p-Value | OR 2 | 95% CI 2 | p-Value | aOR 2 | 95% CI 2 | p-Value |
| Age (years) | 37.6 (20.4) | 44.1 (20.5) | 37.7 (20.4) | 1.02 | (1.00, 1.03) | 0.0093 | 1.02 | [1.00, 1.03] | 0.0015 | |
| Sex | ||||||||||
| Male | 1224 (43.9%) | 35 (51.5%) | 1259 (44.1%) | 0.21 | 1.00 | 1.00 | ||||
| Female | 1565 (56.1%) | 33 (48.5%) | 1598 (55.9%) | 0.74 | (0.46, 1.19) | 0.2149 | 1.07 | [0.53, 2.12] | 0.8572 | |
| Nutritional status | ||||||||||
| Normal weight | 856 (35.8%) | 25 (37.9%) | 881 (35.8%) | <0.001 | 1.00 | 1.00 | ||||
| Underweight | 526 (22.0%) | 28 (42.4%) | 554 (22.5%) | 1.82 | (1.05, 3.16) | 0.0325 | 2.52 | [1.42, 4.47] | 0.0016 | |
| Overweight | 1012 (42.3%) | 13 (19.7%) | 1025 (41.7%) | 0.44 | (0.22, 0.87) | 0.0173 | 0.37 | [0.18, 0.73] | 0.0046 | |
| Smoker | ||||||||||
| No | 2140 (76.7%) | 43 (63.2%) | 2183 (76.4%) | 0.010 | 1.00 | 1.00 | ||||
| Yes | 649 (23.3%) | 25 (36.8%) | 674 (23.6%) | 1.92 | (1.16, 3.16) | 0.0108 | 1.81 | [0.88, 3.74] | 0.1076 | |
| Diabetes mellitus status | ||||||||||
| No or Unknown | 2674 (95.9%) | 61 (89.7%) | 2735 (95.7%) | 0.013 | 1.00 | 1.00 | ||||
| Yes | 115 (4.1%) | 7 (10.3%) | 122 (4.3%) | 2.67 | (1.19, 5.96) | 0.0167 | 2.60 | [1.08, 6.24] | 0.0324 | |
| District type | ||||||||||
| Rural district | 1011 (36.2%) | 20 (29.4%) | 1031 (36.1%) | 0.25 | 1.00 | 1.00 | ||||
| Urban district | 1778 (63.8%) | 48 (70.6%) | 1826 (63.9%) | 1.36 | (0.81, 2.31) | 0.2478 | 1.86 | [1.07, 3.22] | 0.0274 | |
| Type of contact | ||||||||||
| Did not sleep in the same house | 384 (13.8%) | 2 (2.9%) | 386 (13.5%) | 0.010 | 1.00 | 1.00 | ||||
| Slept in same house | 2405 (86.2%) | 66 (97.1%) | 2471(86.5%) | 5.27 | (1.29, 21.60) | 0.0210 | 5.29 | [1.28, 21.89] | 0.0217 | |
| Time since last TB contact | ||||||||||
| <1year | 2385 (85.5%) | 58 (85.3%) | 2443 (85.5%) | 0.21 | 1.00 | 1.00 | ||||
| ≥1 year | 404 (14.5%) | 10 (14.7%) | 414 (14.5%) | 0.87 | (0.52, 2.01) | 0.9593 | 0.87 | [0.43, 1.75] | 0.6978 | |
| Summary Statistics | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No TB Infection N = 1288 1 | TB Infection N = 1083 1 | Total N = 2371 | p-Value | OR 2 | 95% CI 2 | p-Value | aOR 2 | 95% CI 2 | p-Value |
| Age (years) | 36.20 (20.86) | 40.62 (19.42) | 38.22 (20.33) | <0.001 | 1.01 | (1.00, 1.03) | 0.0000 | 1.01 | [1.01, 1.02] | 0.0000 |
| Sex | ||||||||||
| Male | 517 (40.1%) | 497 (45.9%) | 1014 (42.8%) | 0.005 | 1.00 | 1.00 | ||||
| Female | 771 (59.9%) | 586 (54.1%) | 1357 (57.2%) | 0.79 | (0.67, 0.93) | 0.0048 | 0.07 | [0.61, 0.97] | 0.0248 | |
| Nutritional status | ||||||||||
| Normal weight | 405 (35.4%) | 337 (36.1%) | 742 (35.7%) | 0.012 | 1.00 | 1.00 | ||||
| Underweight | 266 (23.2%) | 169 (18.1%) | 435 (20.9%) | 0.76 | (0.60, 0.97) | 0.0282 | 0.89 | [0.69, 1.15] | 0.3729 | |
| Overweight | 474 (41.4%) | 428 (45.8%) | 902 (43.4%) | 1.09 | (0.89, 1.32) | 0.4111 | 1.06 | [0.87, 1.29] | 0.5679 | |
| Smoker | ||||||||||
| No | 1018 (79.0%) | 801 (74.2%) | 1819 (76.7%) | 0.004 | 1.00 | 1.00 | ||||
| Yes | 270 (21.0%) | 282 (26.0%) | 552 (23.3%) | 1.33 | (1.10, 1.61) | 0.036 | 1.06 | [0.81, 1.37] | 0.6887 | |
| Diabetic status | ||||||||||
| No or Unknown | 1239(96.2%) | 1038 (95.8%) | 2277 (96.0%) | 0.66 | 1.00 | 1.00 | ||||
| Yes | 49 (3.8%) | 45 (4.2%) | 94 (4.0%) | 1.10 | (0.73, 1.66) | 0.6629 | 0.80 | [0.51, 1.24] | 0.3043 | |
| District type | ||||||||||
| Rural district | 501 (38.9%) | 355 (32.8%) | 856 (36.1%) | 0.002 | 1.00 | 1.00 | ||||
| Urban district | 787 (61.1%) | 728 (67.2%) | 1515 (63.9%) | 1.31 | (1.10, 1.55) | 0.0020 | 1.18 | [0.99, 1.42] | 0.0318 | |
| Type of contact | ||||||||||
| Did not sleep in the same house | 178 (13.8%) | 140 (12.9%) | 318 (13.4%) | 0.53 | 1.00 | 1.00 | ||||
| Slept in same house | 1110(86.2%) | 943 (87.1%) | 2053 (86.4%) | 1.08 | (0.85, 1.37) | 0.5252 | 1.08 | [0.84, 1.40] | 0.5421 | |
| Time since last TB contact | ||||||||||
| <1 year | 1102 (85.6%) | 946 (87.3%) | 2048 (86.4%) | 0.21 | 1.00 | 1.00 | ||||
| ≥1 year | 186 (14.4%) | 137 (12.7%) | 323 (13.6%) | 0.86 | (0.68, 1.09) | 0.2057 | 0.84 | [0.66, 1.08] | 0.1817 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nababan, B.; Triasih, R.; Chan, G.; Dwihardiani, B.; Hidayat, A.; Dewi, S.C.; Unwanah, L.; Mustofa, A.; du Cros, P. The Yield of Active Tuberculosis Disease and Latent Tuberculosis Infection in Tuberculosis Household Contacts Investigated Using Chest X-ray in Yogyakarta Province, Indonesia. Trop. Med. Infect. Dis. 2024, 9, 34. https://doi.org/10.3390/tropicalmed9020034
Nababan B, Triasih R, Chan G, Dwihardiani B, Hidayat A, Dewi SC, Unwanah L, Mustofa A, du Cros P. The Yield of Active Tuberculosis Disease and Latent Tuberculosis Infection in Tuberculosis Household Contacts Investigated Using Chest X-ray in Yogyakarta Province, Indonesia. Tropical Medicine and Infectious Disease. 2024; 9(2):34. https://doi.org/10.3390/tropicalmed9020034
Chicago/Turabian StyleNababan, Betty, Rina Triasih, Geoffrey Chan, Bintari Dwihardiani, Arif Hidayat, Setyogati C. Dewi, Lana Unwanah, Arif Mustofa, and Philipp du Cros. 2024. "The Yield of Active Tuberculosis Disease and Latent Tuberculosis Infection in Tuberculosis Household Contacts Investigated Using Chest X-ray in Yogyakarta Province, Indonesia" Tropical Medicine and Infectious Disease 9, no. 2: 34. https://doi.org/10.3390/tropicalmed9020034







