Adults with Perinatally Acquired HIV; Emerging Clinical Outcomes and Data Gaps
Abstract
1. Introduction and Epidemiology
2. Cascade of Care
3. Viral Suppression and Acquired Drug Resistance
4. Neurocognitive, Mental Health and Quality of Life outcomes in Adults Living with PaHIV
5. Metabolic and Cardiovascular Health
5.1. Metabolic Health
5.2. Hypertension
5.3. Cardiovascular Disease
6. Respiratory Health
7. Bone Health
8. Sexual and Reproductive Health Needs
8.1. Sexual Health and Contraception
8.2. Cervical Smears and Vaccination
9. Pregnancy in PaHIV
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF. Global and Regional Trends HIV Data. 2023. Available online: https://data.unicef.org/topic/hivaids/global-regional-trends/ (accessed on 6 January 2024).
- UNAIDS. UNAIDS Global HIV Statistics Fact Sheet. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 6 January 2024).
- UNAIDS. The Path That Ends AIDS. 2023 UNAIDS Global AIDS Update. Available online: https://thepath.unaids.org/wp-content/themes/unaids2023/assets/files/2023_report.pdf (accessed on 6 January 2024).
- D’Souza, R.R.; Gopalan, B.P.; Rajnala, N.; Phetsouphanh, C.; Shet, A. Increased monocyte activation with age among HIV-infected long term non-progressor children: Implications for early treatment initiation. HIV Med. 2019, 20, 513–522. [Google Scholar] [CrossRef]
- ISOSS HIV Report. 2022. Available online: https://www.gov.uk/government/publications/infectious-diseases-in-pregnancy-screening-isoss-hiv-report-2022/isoss-hiv-report-2022#vertical-transmissions (accessed on 9 January 2024).
- Sibiude, J.; Le Chenadec, J.; Mandelbrot, L.; Hoctin, A.; Dollfus, C.; Faye, A.; Bui, E.; Pannier, E.; Ghosn, J.; Garrait, V.; et al. Update of Perinatal Human Immunodeficiency Virus Type 1 Transmission in France: Zero Transmission for 5482 Mothers on Continuous Antiretroviral Therapy From Conception and With Undetectable Viral Load at Delivery. Clin. Infect. Dis. 2023, 76, e590–e598. [Google Scholar] [CrossRef] [PubMed]
- Asad, H.; Collins, I.J.; Goodall, R.L.; Crichton, S.; Hill, T.; Doerholt, K.; Foster, C.; Lyall, H.; Post, F.A.; Welch, S.; et al. Mortality and AIDS-defining events among young people following transition from paediatric to adult HIV care in the UK. HIV Med. 2021, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Ayers, S.; McDonald, S.; Frize, G.; Chhabra, S.; Pasvol, T.J.; Fidler, S. Clinical outcomes post transition to adult services in young adults with perinatally acquired HIV infection: Mortality, retention in care and viral suppression. AIDS 2020, 34, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Judd, A.; Davies, M.-A. Adolescent transition among young people with perinatal HIV in high-income and low-income settings. Curr. Opin. HIV AIDS 2018, 13, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Rungmaitree, S.; Thamniamdee, N.; Sachdev, S.; Phongsamart, W.; Lapphra, K.; Wittawatmongkol, O.; Maleesatharn, A.; Khumcha, B.; Hoffman, R.M.; Chokephaibulkit, K. The Outcomes of Transition from Pediatrics to Adult Care among Adolescents and Young Adults with HIV at a Tertiary Care Center in Bangkok. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2022, 21, 232595822211436. [Google Scholar] [CrossRef] [PubMed]
- Mburu, C.; Njuguna, I.; Neary, J.; Mugo, C.; Moraa, H.; Beima-Sofie, K.; Onyango, A.; Oyiengo, L.; Richardson, B.A.; John-Stewart, G.; et al. Mortality and Loss to Follow-Up Among Adolescents and Young Adults Attending HIV Care Programs in Kenya. AIDS Patient Care STDs 2023, 37, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Agwu, A.L.; Lee, L.; Fleishman, J.A.; Voss, C.; Yehia, B.R.; Althoff, K.N.; Rutstein, R.; Mathews, W.C.; Nijhawan, A.; Moore, R.D.; et al. Aging and Loss to Follow-up Among Youth Living With Human Immunodeficiency Virus in the HIV Research Network. J. Adolesc. Health 2015, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.; Agwu, A. Adolescents and young adults with early acquired HIV infection in the united states: Unique challenges in treatment and secondary prevention. Expert Rev. Anti-Infect. Ther. 2021, 19, 457–471. [Google Scholar] [CrossRef]
- Maskew, M.; Technau, K.; Davies, M.-A.; Vreeman, R.; Fox, M.P. Adolescent retention in HIV care within differentiated service-delivery models in sub-Saharan Africa. Lancet HIV 2022, 9, e726–e734. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Sainz, T.; Jimenez de Ory, S.; Bernardino, I.; Díez, C.; Torres, B.; Merino, D.; Iribarren, J.A.; Portilla, I.; Ríos, M.J.; et al. Clinical, Immunological, and Virological Outcomes Among Youths With Perinatal HIV After Transition to Adult Units in Spain From 1997 to 2016. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 86, 240–247. [Google Scholar] [CrossRef]
- Chhabra, S.; Fidler, S.; Ayers, S.; Bower, M.; Lyall, H.; Foster, C. Malignancy and all-cause mortality; incidence in adolescents and young adults living with perinatally acquired HIV. J. Virus Erad. 2020, 6, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Fish, R.; Judd, A.; Jungmann, E.; O’Leary, C.; Foster, C. Mortality in perinatally HIV-infected young people in England following transition to adult care: An HIV Young Persons Network (HYPNet) audit. HIV Med. 2014, 15, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Singtoroj, T.; Teeraananchai, S.; Chokephaibulkit, K.; Phanuphak, N.; Gatechompol, S.; Hansudewechakul, R.; Dang, H.L.D.; Tran, D.N.H.; Kerr, S.; Sohn, A.H.; et al. Factors Associated with Morbidity and Mortality Among Sexually Active Asian Adolescents and Young Adults with Perinatally Acquired HIV. AIDS Res. Hum. Retroviruses 2023, 39, 285–293. [Google Scholar] [CrossRef]
- Neilan, A.M.; Karalius, B.; Patel, K.; Van Dyke, R.B.; Abzug, M.J.; Agwu, A.L.; Williams, P.L.; Purswani, M.; Kacanek, D.; Oleske, J.M.; et al. Association of Risk of Viremia, Immunosuppression, Serious Clinical Events, and Mortality With Increasing Age in Perinatally Human Immunodeficiency Virus–Infected Youth. JAMA Pediatr. 2017, 171, 450–460. [Google Scholar] [CrossRef]
- Neilan, A.M.; Lu, F.; Gebo, K.A.; Diaz-Reyes, R.; Huang, M.; Parker, R.A.; Karalius, B.; Patel, K.; Voss, C.; Ciaranello, A.L.; et al. Higher Acuity Resource Utilization With Older Age and Poorer HIV Control in Adolescents and Young Adults in the HIV Research Network. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 83, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Desmonde, S.; Neilan, A.M.; Musick, B.; Patten, G.; Chokephaibulkit, K.; Edmonds, A.; Duda, S.N.; Malateste, K.; Wools-Kaloustian, K.; Ciaranello, A.L.; et al. Time-varying age- and CD4-stratified rates of mortality and WHO stage 3 and stage 4 events in children, adolescents and youth 0 to 24 years living with perinatally acquired HIV, before and after antiretroviral therapy initiation in the paediatric IeDEA Global Cohort Consortium. J. Int. AIDS Soc. 2020, 23, e25617. [Google Scholar] [CrossRef]
- Ruffieux, Y.; Dhokotera, T.; Muchengeti, M.; Bartels, L.; Olago, V.; Bohlius, J.; Singh, E.; Egger, M.; Rohner, E. Cancer risk in adolescents and young adults living with HIV in South Africa: A nationwide cohort study. Lancet HIV 2021, 8, e614–e622. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Gerver, S.M.; Fidler, S.; Ward, H. Adherence to antiretroviral therapy in adolescents living with HIV. AIDS 2014, 28, 1945–1956. [Google Scholar] [CrossRef]
- Tassiopoulos, K.; Huo, Y.; Kacanek, D.; Malee, K.; Nichols, S.; Mellins, C.A.; Kohlhoff, S.; Van Dyke, R.B. Association of Perceived Social Support with Viral Suppression Among Young Adults with Perinatally-Acquired HIV in the US-based Pediatric HIV/AIDS Cohort Study (PHACS). Clin. Epidemiol. 2023, 15, 601–611. [Google Scholar] [CrossRef]
- Weijsenfeld, A.M.; Smit, C.; Wit, F.W.N.M.; Mudrikova, T.; Nellen, J.F.J.B.; van der Valk, M.; Pajkrt, D. Long-Term Virological Treatment Outcomes in Adolescents and Young Adults With Perinatally and Non-Perinatally Acquired Human Immunodeficiency Virus. Open Forum Infect. Dis. 2022, 9, ofac561. [Google Scholar] [CrossRef]
- Ungaro, R.; Taramasso, L.; Bruzzone, B.; Vicenti, I.; Galli, L.; Borghi, V.; Francisci, D.; Pecorari, M.; Zoncada, A.; Callegaro, A.P.; et al. Prevalence of acquired resistance mutations in a large cohort of perinatally infected HIV-1 patients. Clin. Microbiol. Infect. 2019, 25, 1443–1446. [Google Scholar] [CrossRef]
- Beltrán-Pavez, C.; Gutiérrez-López, M.; Rubio-Garrido, M.; Valadés-Alcaraz, A.; Prieto, L.; Ramos, J.T.; Jiménez De Ory, S.; Navarro, M.; Díez-Romero, C.; Pulido, F.; et al. Virological outcome among HIV infected patients transferred from pediatric care to adult units in Madrid, Spain (1997–2017). Sci. Rep. 2020, 10, 16891. [Google Scholar] [CrossRef] [PubMed]
- Osmundo, G.d.S.; da Costa, R.A.; Ruocco, R.M.A.; Francisco, R.P.V. Pregnancy in women living with perinatally acquired HIV: Perinatal outcomes and drug resistance profile. Clinics 2023, 78, 100174. [Google Scholar] [CrossRef]
- Penda, C.I.; Moukoko Mbonjo, M.; Fokam, J.; Djeuda, A.B.D.; Grace, N.; Ateba Ndongo, F.; Bilong, S.; Eyoum Bille, B.; Koki Ndombo, P.; Aghokeng, A.; et al. Rate of virological failure and HIV-1 drug resistance among HIV-infected adolescents in routine follow-up on health facilities in Cameroon. PLoS ONE 2022, 17, e0276730. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Ayers, S.; Fidler, S. Antiretroviral adherence for adolescents growing up with HIV: Understanding real life, drug delivery and forgiveness. Ther. Adv. Infect. Dis. 2020, 7, 204993612092017. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.T.; Boubour, A.; Saylor, D.; Das, M.; Bearden, D.R.; Birbeck, G.L. Global HIV neurology. AIDS 2019, 33, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Cotton, M.F.; Violari, A.; Otwombe, K.; Panchia, R.; Dobbels, E.; Rabie, H.; Josipovic, D.; Liberty, A.; Lazarus, E.; Innes, S.; et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: Results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013, 382, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Laughton, B.; Cornell, M.; Grove, D.; Kidd, M.; Springer, P.E.; Dobbels, E.; van Rensburg, A.J.; Violari, A.; Babiker, A.G.; Madhi, S.A.; et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS 2012, 26, 1685–1690. [Google Scholar] [CrossRef]
- Jantarabenjakul, W.; Chonchaiya, W.; Puthanakit, T.; Theerawit, T.; Payapanon, J.; Sophonphan, J.; Veeravigom, M.; Jahanshad, N.; Thompson, P.M.; Ananworanich, J.; et al. Low risk of neurodevelopmental impairment among perinatally acquired HIV-infected preschool children who received early antiretroviral treatment in Thailand. J. Int. AIDS Soc. 2019, 22, e25278. [Google Scholar] [CrossRef]
- Benki-Nugent, S.; Tamasha, N.; Mueni, A.; Laboso, T.; Wamalwa, D.C.; Njuguna, I.; Gómez, L.; Tapia, K.; Bangirana, P.; Maleche-Obimbo, E.; et al. Early Antiretroviral Therapy Reduces Severity but Does Not Eliminate Neurodevelopmental Compromise in Children With HIV. JAIDS J. Acquir. Immune Defic. Syndr. 2023, 93, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Malee, K.M.; Chernoff, M.C.; Sirois, P.A.; Williams, P.L.; Garvie, P.A.; Kammerer, B.L.; Harris, L.L.; Nozyce, M.L.; Yildirim, C.; Nichols, S.L. Impact of Perinatally Acquired HIV Disease Upon Longitudinal Changes in Memory and Executive Functioning. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 75, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Judd, A.; Le Prevost, M.; Melvin, D.; Arenas-Pinto, A.; Parrott, F.; Winston, A.; Foster, C.; Sturgeon, K.; Rowson, K.; Gibb, D.M. Cognitive Function in Young Persons With and Without Perinatal HIV in the AALPHI Cohort in England: Role of Non–HIV-Related Factors. Clin. Infect. Dis. 2016, 63, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.N.; Zimmerman, R.; Korich, R.; Raymond, J.; Dolezal, C.; Choi, C.J.; Leu, C.S.; Nguyen, N.; Malee, K.; Wiznia, A.; et al. Longitudinal trajectories of neurocognitive test performance among individuals with perinatal HIV-infection and -exposure: Adolescence through young adulthood. AIDS Care 2020, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Coutifaris, P.; Byrd, D.; Childs, J.; Clark, U.; Posada, R.; Robbins, R.; Morgello, S. Neurobehavioral outcomes in young adults with perinatally acquired HIV. AIDS 2020, 34, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.L. Central Nervous System Impact of Perinatally Acquired HIV in Adolescents and Adults: An Update. Curr. HIV/AIDS Rep. 2022, 19, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Pinto, A.; Judd, A.; Melvin, D.; Le Prevost, M.; Foster, C.; Sturgeon, K.; Winston, A.; Thompson, L.C.; Gibb, D.M.; Castro, H. Learning and memory function in young people with and without perinatal HIV in England. PLoS ONE 2022, 17, e0273645. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.; Cantos, A.; Ramon, C.V.; Shen, Y.; Shah, J.; Jang, G.; Baccarelli, A.A.; Arpadi, S.M.; Yin, M.T. Epigenetic Age in Young African American Adults With Perinatally Acquired HIV. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 87, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Sanjeeva, G.N.; Sahana, M.; Pavithra, H.B.; Swamy, V.H.T.; Srirama, B.R.; Sunil Kumar, D.R.; Hande, L.; Mothi, S.N. Transition of Children with Perinatally Acquired HIV-Infection into Adulthood: Social Outcome and Quality of Life. Indian J. Pediatr. 2019, 86, 233–240. [Google Scholar] [CrossRef]
- Sirois, P.A.; Huo, Y.; Nozyce, M.L.; Garvie, P.A.; Harris, L.L.; Malee, K.; McEvoy, R.; Mellins, C.A.; Nichols, S.L.; Smith, R.; et al. Ageing with HIV: A longitudinal study of markers of resilience in young adults with perinatal exposure to HIV, with or without perinatally acquired HIV. J. Int. AIDS Soc. 2022, 25, e25982. [Google Scholar] [CrossRef]
- Abrams, E.J.; Mellins, C.A.; Bucek, A.; Dolezal, C.; Raymond, J.; Wiznia, A.; Jurgrau, A.; Bamji, M.; Leu, C.-S.; Ng, Y.K.W. Behavioral Health and Adult Milestones in Young Adults With Perinatal HIV Infection or Exposure. Pediatrics 2018, 142, e20180938. [Google Scholar] [CrossRef] [PubMed]
- Karugaba, G.; Thupayagale-Tshweneagae, G.; Moleki, M.M.; Mabikwa, O.V.; Matshaba, M. Determinants of health-related quality of life in young adults living with perinatally acquired HIV infection in Botswana. S. Afr. J. HIV Med. 2022, 23, a1362. [Google Scholar] [CrossRef] [PubMed]
- Karugaba, G.; Thupayagale-Tshweneagae, G.; Moleki, M.M.; Matshaba, M. Challenges and coping strategies among young adults living with perinatally acquired HIV infection in Botswana. A qualitative study. PLoS ONE 2023, 18, e0284467. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Radua, J.; Olivola, M.; Croce, E.; Soardo, L.; Salazar de Pablo, G.; Il Shin, J.; Kirkbride, J.B.; Jones, P.; Kim, J.H.; et al. Age at onset of mental disorders worldwide: Large-scale meta-analysis of 192 epidemiological studies. Mol. Psychiatry 2022, 27, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Too, E.K.; Abubakar, A.; Nasambu, C.; Koot, H.M.; Cuijpers, P.; Newton, C.R.J.C.; Nyongesa, M.K. Prevalence and factors associated with common mental disorders in young people living with HIV in sub-Saharan Africa: A systematic review. J. Int. AIDS Soc. 2021, 24, e25705. [Google Scholar] [CrossRef] [PubMed]
- Nyongesa, M.K.; Mwangi, P.; Kinuthia, M.; Hassan, A.S.; Koot, H.M.; Cuijpers, P.; Newton, C.R.J.C.; Abubakar, A. Prevalence, risk and protective indicators of common mental disorders among young people living with HIV compared to their uninfected peers from the Kenyan coast: A cross-sectional study. BMC Psychiatry 2021, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Durteste, M.; Kyselyova, G.; Volokha, A.; Judd, A.; Thorne, C.; Cortina-Borja, M.; Malyuta, R.; Martsynovska, V.; Nizova, N.; Bailey, H. Anxiety symptoms and felt stigma among young people living with perinatally or behaviourally-acquired HIV in Ukraine: A cross-sectional survey. PLoS ONE 2019, 14, e0210412. [Google Scholar] [CrossRef] [PubMed]
- Chantaratin, S.; Trimetha, K.; Werarak, P.; Lapphra, K.; Maleesatharn, A.; Rungmaitree, S.; Wittawatmongkol, O.; Phongsamart, W.; Kongstan, N.; Khumcha, B.; et al. Depression and Anxiety in Youth and Young Adults Living with HIV: Frequency and Associated Factors in Thai Setting. J. Int. Assoc. Provid. AIDS Care (JIAPAC) 2022, 21, 232595822211018. [Google Scholar] [CrossRef]
- Bucek, A.; Mellins, C.A.; Leu, C.-S.; Dolezal, C.; Korich, R.; Wiznia, A.; Abrams, E.J. Psychiatric disorders and young adult milestones in HIV-exposed, uninfected youth. AIDS Care 2020, 32, 420–428. [Google Scholar] [CrossRef]
- Le Prevost, M.; Arenas-Pinto, A.; Melvin, D.; Parrott, F.; Foster, C.; Ford, D.; Evangeli, M.; Winston, A.; Sturgeon, K.; Rowson, K.; et al. Anxiety and depression symptoms in young people with perinatally acquired HIV and HIV affected young people in England. AIDS Care 2018, 30, 1040–1049. [Google Scholar] [CrossRef]
- Kang, E.; Mellins, C.A.; Kim, W.; Dolezal, C.; Kindler, C.; Leu, C.-S.; Abrams, E.J. Navigating Stigma Trajectory and Mental Health Among Young Adults Living with Perinatal HIV in New York City. AIDS Behav. 2021, 25, 3712–3720. [Google Scholar] [CrossRef] [PubMed]
- Aurpibul, L.; Tangmunkongvorakul, A.; Detsakunathiwatchara, C.; Masurin, S.; Srita, A.; Meeart, P.; Chueakong, W. Social effects of HIV disclosure, an ongoing challenge in young adults living with perinatal HIV: A qualitative study. Front. Public Health 2023, 11, 1150419. [Google Scholar] [CrossRef] [PubMed]
- Kidman, R.; Violari, A. Growing up positive: Adolescent HIV disclosure to sexual partners and others. AIDS Care 2020, 32, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Evangeli, M.; Foster, C.; Musiime, V.; Fidler, S.; Seeley, J.; Gnan, G. A randomised feasibility trial of an intervention to support sharing of HIV status for 18–25-year olds living with perinatally acquired HIV compared with standard care: HIV Empowering Adults’ Decisions to Share—UK/Uganda Project (HEADS-UP). Pilot Feasibility Stud. 2020, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Kreniske, P.; Mellins, C.A.; Dolezal, C.; Morrison, C.; Shea, E.; Fisher, P.W.; Kluisza, L.; Robbins, R.N.; Nguyen, N.; Leu, C.-S.; et al. Predictors of Attempted Suicide Among Youth Living With Perinatal HIV Infection and Perinatal HIV-Exposed Uninfected Counterparts. JAIDS J. Acquir. Immune Defic. Syndr. 2021, 88, 348–355. [Google Scholar] [CrossRef]
- Liotta, L.; Kluisza, L.; Nguyen, N.; Leu, C.-S.; Levine, A.; Snyder, C.; Robbins, R.; Dolezal, C.; Kreniske, P.; Wiznia, A.; et al. Hope for the future protects against suicidal ideation among adolescents and young adults affected by perinatal HIV. AIDS Care 2023, 35, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Kreniske, P.; Morrison, C.; Spencer, B.H.; Levine, A.; Liotta, L.; Fisher, P.W.; Nguyen, N.; Robbins, R.N.; Dolezal, C.; Kluisza, L.; et al. HIV and suicide risk across adolescence and young adulthood: An examination of socio-demographic, contextual and psychosocial risk factors for attempted suicide in a longitudinal cohort of ageing adolescents affected by HIV living in the New York City Area. J. Int. AIDS Soc. 2022, 25, e25984. [Google Scholar] [CrossRef] [PubMed]
- Aurpibul, L.; Kosalaraksa, P.; Kawichai, S.; Lumbiganon, P.; Ounchanum, P.; Natalie Songtaweesin, W.; Sudjaritruk, T.; Chokephaibulkit, K.; Rungmaitree, S.; Suwanlerk, T.; et al. Alcohol use, suicidality and virologic non-suppression among young adults with perinatally acquired HIV in Thailand: A cross-sectional study. J. Int. AIDS Soc. 2023, 26, e26064. [Google Scholar] [CrossRef]
- Mallik, I.; Pasvol, T.; Frize, G.; Ayres, S.; Barrera, A.; Fidler, S.; Foster, C. Psychotic disorders in young adults with perinatally acquired HIV: A UK case series. Psychol. Med. 2022, 52, 2263–2269. [Google Scholar] [CrossRef]
- Bucek, A.; Leu, C.-S.; Benson, S.; Warne, P.; Abrams, E.J.; Elkington, K.S.; Dolezal, C.; Wiznia, A.; Mellins, C.A. Psychiatric Disorders, Antiretroviral Medication Adherence and Viremia in a Cohort of Perinatally HIV-Infected Adolescents and Young Adults. Pediatr. Infect. Dis. J. 2018, 37, 673–677. [Google Scholar] [CrossRef]
- Benson, S.; Elkington, K.S.; Leu, C.-S.; Bucek, A.; Dolezal, C.; Warne, P.; Mellins, C. Association Between Psychiatric Disorders, Substance Use, and Sexual Risk Behaviors in Perinatally HIV-Exposed Youth. J. Assoc. Nurses AIDS Care 2018, 29, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Elkington, K.S.; Bauermeister, J.A.; Santamaria, E.K.; Dolezal, C.; Mellins, C.A. Substance Use and the Development of Sexual Risk Behaviors in Youth Perinatally Exposed to HIV. J. Pediatr. Psychol. 2015, 40, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Attoh-Okine, N.-D.; Corbeil, T.; Poku, O.; Kluisza, L.; Liotta, L.; Morrison, C.; Dolezal, C.; Robbins, R.N.; Kreniske, P.; Abrams, E.J.; et al. Prevalence and Correlates of Intimate Partner Violence Victimization Among Urban Adolescents and Young Adults Living With Perinatally-Acquired HIV Infection or Perinatal HIV Exposure. JAIDS J. Acquir. Immune Defic. Syndr. 2024, 95, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Kidman, R.; Violari, A. Dating Violence Against HIV-Infected Youth in South Africa: Associations With Sexual Risk Behavior, Medication Adherence, and Mental Health. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 77, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Merrill, K.G.; Campbell, J.C.; Decker, M.R.; McGready, J.; Burke, V.M.; Mwansa, J.K.; Miti, S.; Frimpong, C.; Kennedy, C.E.; Denison, J.A. Past-Year Violence Victimization is Associated with Viral Load Failure Among HIV-Positive Adolescents and Young Adults. AIDS Behav. 2021, 25, 1373–1383. [Google Scholar] [CrossRef]
- Koethe, J.R.; Jenkins, C.A.; Lau, B.; Shepherd, B.E.; Justice, A.C.; Tate, J.P.; Buchacz, K.; Napravnik, S.; Mayor, A.M.; Horberg, M.A.; et al. Rising Obesity Prevalence and Weight Gain Among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res. Hum. Retroviruses 2016, 32, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bailin, S.S.; Gabriel, C.L.; Wanjalla, C.N.; Koethe, J.R. Obesity and Weight Gain in Persons with HIV. Curr. HIV/AIDS Rep. 2020, 17, 138–150. [Google Scholar] [CrossRef]
- Sahagun, S.J.; Yeramosu, T.; Purdy, J.B.; Reynolds, J.C.; Hadigan, C.M. Associations Between Central Obesity and Lifelong Antiviral Therapy in Adults Living With HIV Acquired From Early Childhood. JAIDS J. Acquir. Immune Defic. Syndr. 2022, 89, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Arrive, E.; Viard, J.-P.; Salanave, B.; Dollfus, C.; Matheron, S.; Reliquet, V.; Arezes, E.; Nailler, L.; Vigouroux, C.; Warszawski, J. Metabolic risk factors in young adults infected with HIV since childhood compared with the general population. PLoS ONE 2018, 13, e0206745. [Google Scholar] [CrossRef]
- Aurpibul, L.; Namwongprom, S.; Sudjaritruk, T.; Ounjaijean, S. Metabolic syndrome, biochemical markers, and body composition in youth living with perinatal HIV infection on antiretroviral treatment. PLoS ONE 2020, 15, e0230707. [Google Scholar] [CrossRef]
- Foster, C.; Smith, C.; Henderson, M.; Cheung, M.; Fidler, S. Metabolic Health and Bone Density in Youth Living with Perinatal HIV. CROI, 19–22 February 2023, Seattle, Washington. Poster Session Q4 (822). Available online: https://www.natap.org/2023/CROI/croi_175.htm (accessed on 6 January 2024).
- Venter, W.D.F.; Moorhouse, M.; Sokhela, S.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef]
- Koay, W.L.A.; Dirajlal-Fargo, S.; Levy, M.E.; Kulie, P.; Monroe, A.; Castel, A.D.; Rakhmanina, N.Y.; D’Angelo, L.; Rakhmanina, N.; Kharfen, M.; et al. Integrase Strand Transfer Inhibitors and Weight Gain in Children and Youth With Perinatal Human Immunodeficiency Virus in the DC Cohort. Open Forum Infect. Dis. 2021, 8, ofab308. [Google Scholar] [CrossRef]
- Rose, P.C.; De la Rey Nel, E.; Cotton, M.F.; Otwombe, K.; Browne, S.H.; Frigati, L.J.; Rabie, H.; Innes, S. Decreased Hepatic Steatosis in South African Adolescents With Perinatal HIV Switching to Dolutegravir-containing Regimens. Pediatr. Infect. Dis. J. 2023, 42, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; Di Biagio, A.; Bovis, F.; Forlanini, F.; Albani, E.; Papaioannu, R.; Giacomet, V. Switching to Integrase Inhibitors Unlinked to Weight Increase in Perinatally HIV-Infected Young Adults and Adolescents: A 10-Year Observational Study. Microorganisms 2020, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Kalligeros, M.; Vassilopoulos, A.; Shehadeh, F.; Vassilopoulos, S.; Lazaridou, I.; Mylonakis, E.; Promrat, K.; Wands, J.R. Prevalence and Characteristics of Nonalcoholic Fatty Liver Disease and Fibrosis in People Living With HIV Monoinfection: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 1708–1722. [Google Scholar] [CrossRef]
- Aepfelbacher, J.A.; Balmaceda, J.; Purdy, J.; Mattingly, A.; Zambell, K.; Hawkins, K.; Chairez, C.; Curl, K.A.; Dee, N.; Hadigan, C. Increased Prevalence of Hepatic Steatosis in Young Adults With Lifelong HIV. J. Infect. Dis. 2019, 220, 266–269. [Google Scholar] [CrossRef]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Fahme, S.A.; Bloomfield, G.S.; Peck, R. Hypertension in HIV-Infected Adults. Hypertension 2018, 72, 44–55. [Google Scholar] [CrossRef]
- O’Neil, P.J.; Stafford, K.A.; Ryscavage, P.A. Assessing risk factors for hypertension in young adults with perinatally acquired HIV infection: A case–control study. HIV Med. 2022, 23, 457–464. [Google Scholar] [CrossRef]
- Ryscavage, P.; Still, W.; Nyemba, V.; Stafford, K. Prevalence of Systemic Hypertension Among HIV-Infected and HIV-Uninfected Young Adults in Baltimore, Maryland. South. Med. J. 2019, 112, 387–391. [Google Scholar] [CrossRef]
- Kamkuemah, M.; Gausi, B.; Oni, T. Missed opportunities for NCD multimorbidity prevention in adolescents and youth living with HIV in urban South Africa. BMC Public Health 2020, 20, 821. [Google Scholar] [CrossRef] [PubMed]
- Sainz, T.; Álvarez-Fuente, M.; Navarro, M.L.; Díaz, L.; Rojo, P.; Blázquez, D.; Isabel de José, M.; Ramos, J.T.; Serrano-Villar, S.; Martínez, J.; et al. Subclinical Atherosclerosis and Markers of Immune Activation in HIV-Infected Children and Adolescents. JAIDS J. Acquir. Immune Defic. Syndr. 2014, 65, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Chatterton-Kirchmeier, S.; Camacho-Gonzalez, A.F.; McCracken, C.E.; Chakraborty, R.; Batisky, D.L. Increased Prevalence of Elevated Blood Pressures in HIV-Infected Children, Adolescents and Young Adults. Pediatr. Infect. Dis. J. 2015, 34, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, H.E.; Griffith, D.; Agwu, A.L. Preventing and diagnosing HIV-related comorbidities in adolescents. Top. Antivir. Med. 2022, 30, 537–544. [Google Scholar] [PubMed]
- Hanna, D.B.; Guo, M.; Bůžková, P.; Miller, T.L.; Post, W.S.; Stein, J.H.; Currier, J.S.; Kronmal, R.A.; Freiberg, M.S.; Bennett, S.N.; et al. HIV Infection and Carotid Artery Intima-media Thickness: Pooled Analyses Across 5 Cohorts of the NHLBI HIV-CVD Collaborative. Clin. Infect. Dis. 2016, 63, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elmoniem, K.Z.; Unsal, A.B.; Eshera, S.; Matta, J.R.; Muldoon, N.; McAreavey, D.; Purdy, J.B.; Hazra, R.; Hadigan, C.; Gharib, A.M. Increased Coronary Vessel Wall Thickness in HIV-Infected Young Adults. Clin. Infect. Dis. 2014, 59, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Bavinger, C.; Bendavid, E.; Niehaus, K.; Olshen, R.A.; Olkin, I.; Sundaram, V.; Wein, N.; Holodniy, M.; Hou, N.; Owens, D.K.; et al. Risk of Cardiovascular Disease from Antiretroviral Therapy for HIV: A Systematic Review. PLoS ONE 2013, 8, e59551. [Google Scholar] [CrossRef] [PubMed]
- Worm, S.W.; Sabin, C.; Weber, R.; Reiss, P.; El-Sadr, W.; Dabis, F.; De Wit, S.; Law, M.; Monforte Antonella, D.A.; Friis-Møller, N.; et al. Risk of Myocardial Infarction in Patients with HIV Infection Exposed to Specific Individual Antiretroviral Drugs from the 3 Major Drug Classes: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. J. Infect. Dis. 2010, 201, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Hsue, P.Y.; Hunt, P.W.; Wu, Y.; Schnell, A.; Ho, J.E.; Hatano, H.; Xie, Y.; Martin, J.N.; Ganz, P.; Deeks, S.G. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS 2009, 23, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.D.; Sullam, P.M.; Stoddart, C.A.; McCune, J.M. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. AIDS 2011, 25, 2243–2248. [Google Scholar] [CrossRef]
- Jensen, B.V.; Skovsgaard, T.; Nielsen, S.L. Functional monitoring of anthracycline cardiotoxicity:a prospective, blinded, long-term observational study of outcome in 120 patients. Ann. Oncol. 2002, 13, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Rylance, J.; McHugh, G.; Metcalfe, J.; Mujuru, H.; Nathoo, K.; Wilmore, S.; Rowland-Jones, S.; Majonga, E.; Kranzer, K.; Ferrand, R.A. Chronic lung disease in HIV-infected children established on antiretroviral therapy. AIDS 2016, 30, 2795–2803. [Google Scholar] [CrossRef]
- Zar, H.J. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr. Pulmonol. 2008, 43, 1–10. [Google Scholar] [CrossRef]
- Ferrand, R.A.; Desai, S.R.; Hopkins, C.; Elston, C.M.; Copley, S.J.; Nathoo, K.; Ndhlovu, C.E.; Munyati, S.; Barker, R.D.; Miller, R.F.; et al. Chronic Lung Disease in Adolescents With Delayed Diagnosis of Vertically Acquired HIV Infection. Clin. Infect. Dis. 2012, 55, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.R.; Nair, A.; Rylance, J.; Mujuru, H.; Nathoo, K.; McHugh, G.; Majonga, E.; Metcalfe, J.; Kranzer, K.; Ferrand, R.A. Human Immunodeficiency Virus-Associated Chronic Lung Disease in Children and Adolescents in Zimbabwe: Chest Radiographic and High-Resolution Computed Tomographic Findings. Clin. Infect. Dis. 2018, 66, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.K.; Shackley, F.; Ugonna, K.; Ryan, C.E.; Hughes, D.; Owens, S.; Brown, P.; Riordan, A.; Ilozue, C.I.; Rowland-Jones, S.L.; et al. Chronic Lung Disease in Patients With Perinatally Acquired HIV in England: A Retrospective Case-Note Review. Pediatr. Infect. Dis. J. 2020, 39, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Shearer, W.T.; Jacobson, D.L.; Yu, W.; Siberry, G.K.; Purswani, M.; Siminski, S.; Butler, L.; Leister, E.; Scott, G.; Van Dyke, R.B.; et al. Long-term pulmonary complications in perinatally HIV-infected youth. J. Allergy Clin. Immunol. 2017, 140, 1101–1111.e1107. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.J.; Krishnan-Sarin, S.; Exil, V.J.; Hamburg, N.M.; Fetterman, J.L.; Ichinose, F.; Perez-Pinzon, M.A.; Rezk-Hanna, M.; Williamson, E. Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 703–728. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.A.; Gentzke, A.S.; Sawdey, M.D.; Chang, J.T.; Anic, G.M.; Wang, T.W.; Creamer, M.R.; Jamal, A.; Ambrose, B.K.; King, B.A. e-Cigarette Use Among Youth in the United States, 2019. JAMA 2019, 322, 2095–2103. [Google Scholar] [CrossRef]
- EACS Guidelines V12. 2023. Available online: https://www.eacsociety.org/media/guidelines-12.0.pdf (accessed on 7 February 2024).
- Chevalley, T.; Rizzoli, R. Acquisition of peak bone mass. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101616. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Wang, J.; Siberry, G.K.; Miller, T.L.; Geffner, M.E.; Hazra, R.; Borkowsky, W.; Chen, J.S.; Dooley, L.; Patel, K.; et al. Bone mineral density in children and adolescents with perinatal HIV infection. AIDS 2013, 27, 211–220. [Google Scholar] [CrossRef]
- Sudjaritruk, T.; Bunupuradah, T.; Aurpibul, L.; Kosalaraksa, P.; Kurniati, N.; Prasitsuebsai, W.; Sophonphan, J.; Sohn, A.H.; Ananworanich, J.; Puthanakit, T.; et al. Adverse bone health and abnormal bone turnover among perinatally HIV-infected Asian adolescents with virological suppression. HIV Med. 2017, 18, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.L.; Hartley, A.; Majonga, E.; McHugh, G.; Crabtree, N.; Rukuni, R.; Bandason, T.; Mukwasi-Kahari, C.; Ward, K.A.; Mujuru, H.; et al. Older age at initiation of antiretroviral therapy predicts low bone mineral density in children with perinatally-infected HIV in Zimbabwe. Bone 2019, 125, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Schtscherbyna, A.; Pinheiro, M.F.M.C.; Mendonça, L.M.C.d.; Gouveia, C.; Luiz, R.R.; Machado, E.S.; Farias, M.L.F.d. Factors associated with low bone mineral density in a Brazilian cohort of vertically HIV-infected adolescents. Int. J. Infect. Dis. 2012, 16, e872–e878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rehman, A.M.; Kowo-Nyakoko, F.; Rukuni, R.; Rehman, A.M.; Mukwasi-Kahari, C.; Madanhire, T.; Kowo-Nyakoko, F.; McHugh, G.; Filteau, S.; Chipanga, J.; et al. Effect of HIV infection on growth and bone density in peripubertal children in the era of antiretroviral therapy: A cross-sectional study in Zimbabwe. Lancet Child Adolesc Health 2021, 5, 569–581. [Google Scholar] [CrossRef]
- Henderson, M.S.C.; Fidler, S.; Cheung, M.; Foster, C. The BONDY Study: Bone Density in Youth Living with Perinatally-Acquired HIV. CROI, 19–22 February 2023, Seattle, Washington. Poster Session Q4 (823). Available online: https://www.croiconference.org/abstract/the-bondy-study-bone-density-in-youth-living-with-perinatally-acquired-hiv/ (accessed on 6 January 2024).
- Arpadi, S.M.; Shiau, S.; Marx-Arpadi, C.; Yin, M.T. Bone health in HIV-infected children, adolescents and young adults: A systematic review. J. AIDS Clin. Res. 2014, 5, 374. [Google Scholar] [CrossRef] [PubMed]
- Innes, S.; Patel, K. Noncommunicable diseases in adolescents with perinatally acquired HIV-1 infection in high-income and low-income settings. Curr. Opin. HIV AIDS 2018, 13, 187–195. [Google Scholar] [CrossRef] [PubMed]
- British HIV Association. BHIVA Guidelines on Antiretroviral Treatment for Adults Living with HIV-1 2022 (2023 Interim Update). Available online: https://www.bhiva.org/file/63513a1745ea9/BHIVA-guidelines-on-antiretroviral-treatment-for-adults-living-with-HIV-1-2022.pdf (accessed on 7 February 2024).
- Nguyen, H.S.; Van Tran, K.; Chen, S.-Y.; Tam, K.-W. A Systematic Review and Meta-Analysis of Randomized Controlled Trials of the Effects of Vitamin D Supplementation on Children and Young Adults with HIV Infection. J. Nutr. 2023, 153, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Sudjaritruk, T.; Bunupuradah, T.; Aurpibul, L.; Kanjanavanit, S.; Chotecharoentanan, T.; Sricharoen, N.; Ounchanum, P.; Suntarattiwong, P.; Pornpaisalsakul, K.; Puthanakit, T. Impact of Vitamin D and Calcium Supplementation on Bone Mineral Density and Bone Metabolism Among Thai Adolescents With Perinatally Acquired Human Immunodeficiency Virus (HIV) Infection: A Randomized Clinical Trial. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, 1555–1564. [Google Scholar] [CrossRef]
- Lindsey, J.C.; Jacobson, D.L.; Spiegel, H.M.; Gordon, C.M.; Hazra, R.; Siberry, G.K. Safety and Efficacy of 48 and 96 Weeks of Alendronate in Children and Adolescents With Perinatal Human Immunodeficiency Virus Infection and Low Bone Mineral Density for Age. Clin. Infect. Dis. 2021, 72, 1059–1063. [Google Scholar] [CrossRef]
- Nott, V.R.; Hazell, G.A.; Ayres, S.; Kirkhope, N.; Fidler, S.; Foster, C. Sexual and reproductive health needs of young women living with perinatally acquired HIV. Int. J. STD AIDS 2023, 34, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Judd, A.; Foster, C.; Thompson, L.C.; Sturgeon, K.; Le Prevost, M.; Jungmann, E.; Rowson, K.; Castro, H.; Gibb, D.M. Sexual health of young people with perinatal HIV and HIV negative young people in England. PLoS ONE 2018, 13, e0205597. [Google Scholar] [CrossRef] [PubMed]
- Croucher, A.P.; Jose, S.; McDonald, S.; Foster, C.; Fidler, S. Sexual and reproductive health in a UK cohort of young adults perinatally infected with HIV: Table 1. Sex. Transm. Infect. 2013, 89, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Stringer, E.M.; Kendall, M.A.; Lockman, S.; Campbell, T.B.; Nielsen-Saines, K.; Sawe, F.; Cu-uvin, S.; Wu, X.; Currier, J.S. Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS ONE 2018, 13, e0199555. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Sharma, M.; Tan, N.; Barnabas, R.V. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018, 32, 795–808. [Google Scholar] [CrossRef]
- Phanuphak, N.; Teeraananchai, S.; Hansudewechakul, R.; Gatechompol, S.; Chokephaibulkit, K.; Dang, H.L.D.; Tran, D.N.H.; Achalapong, J.; Teeratakulpisarn, N.; Chalermchockcharoenkit, A.; et al. Incidence and Persistence of High-risk Anogenital Human Papillomavirus Infection Among Female Youth With and Without Perinatally Acquired Human Immunodefiency Virus Infection: A 3-year Observational Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, e270–e280. [Google Scholar] [CrossRef] [PubMed]
- Ounchanum, P.; Ounchanum, P.; Achalapong, J.; Achalapong, J.; Teeraananchai, S.; Teeraananchai, S.; Gatechompol, S.; Gatechompol, S.; Phongsamart, W.; Phongsamart, W.; et al. The effects of bivalent human papillomavirus (HPV) vaccination on high-risk anogenital HPV infection among sexually active female adolescents with and without perinatally acquired HIV. Sex. Health 2023, 21, 10–1071. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.; Henderson, M.; Crook, E.; Ayres, S.; Beddows, S.; Panwar, K.; Wright, C.; Lyons, D.; Cowen, M.; Smith, D.; et al. High-risk HPV prevalence and serostatus in women living with perinatally acquired HIV (the SHiP study). Oral Abstracts. HIV Med. 2023, 24, 3–16. [Google Scholar] [CrossRef]
- Phanuphak, N.; Teeratakulpisarn, N.; Pankam, T.; Kerr, S.J.; Barisri, J.; Deesua, A.; Rodbamrung, P.; Hongchookiat, P.; Chomchey, N.; Phanuphak, P.; et al. Anal Human Papillomavirus Infection Among Thai Men Who Have Sex With Men With and Without HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2013, 63, 472–479. [Google Scholar] [CrossRef]
- Gatechompol, S.; Teeratakulpisarn, N.; Wittawatmongkol, O.; Teeraananchai, S.; Kerr, S.J.; Chalermchockcharoenkit, A.; Thamkhantho, M.; Singtoroj, T.; Phanuphak, N.; Sohn, A.H.; et al. Incidence, Persistence, and Factors Associated With HPV Infection Among Male Adolescents With and Without Perinatally Acquired HIV Infection. J. Acquir. Immune Defic. Syndr. 2020, 85, 553–560. [Google Scholar] [CrossRef]
- Byrne, L.; Sconza, R.; Foster, C.; Tookey, P.A.; Cortina-Borja, M.; Thorne, C. Pregnancy incidence and outcomes in women with perinatal HIV infection. AIDS 2017, 31, 1745–1754. [Google Scholar] [CrossRef]
- Pasvol, T.J.; Teh, J.; Balfoussia, D.; Hall, R.; Petersen, C.; Khan, M.; Ayres, S.; Jayasena, C.N.; Foster, C.; Fidler, S. Outcomes of fertility investigations in a cohort of adults with perinatally acquired HIV-1 infection: A UK cross-sectional observational study. AIDS 2021, 35, 343–345. [Google Scholar] [CrossRef]
- Berhie, S.; Yee, L.; Jao, J. The Reproductive Years of Women with Perinatally Acquired HIV. Infect. Dis. Clin. N. Am. 2019, 33, 817–833. [Google Scholar] [CrossRef]
- Trahan, M.-J.; Boucher, M.; Renaud, C.; Karatzios, C.; Metras, M.-E.; Valois, S.; Ransy, D.G.; Lamarre, V.; Kakkar, F. Pregnancies Among the First Generation of Survivors of Perinatal HIV Infection. J. Obstet. Gynaecol. Can. 2020, 42, 446–452. [Google Scholar] [CrossRef]
- Slogrove, A.L.; Bovu, A.; de Beer, S.; Phelanyane, F.; Williams, P.L.; Heekes, A.; Kalk, E.; Mehta, U.; Theron, G.; Abrams, E.J.; et al. Maternal and birth outcomes in pregnant people with and without HIV in the Western Cape, South Africa. AIDS 2024, 38, 59–67. [Google Scholar] [CrossRef]
- Murray, C.; Portwood, C.; Sexton, H.; Kumarendran, M.; Brandon, Z.; Kirtley, S.; Hemelaar, J. Adverse perinatal outcomes attributable to HIV in sub-Saharan Africa from 1990 to 2020: Systematic review and meta-analyses. Commun. Med. 2023, 3, 103. [Google Scholar] [CrossRef]
- Ganchimeg, T.; Ota, E.; Morisaki, N.; Laopaiboon, M.; Lumbiganon, P.; Zhang, J.; Yamdamsuren, B.; Temmerman, M.; Say, L.; Tunçalp, Ö.; et al. Pregnancy and childbirth outcomes among adolescent mothers: A World Health Organization multicountry study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 40–48. [Google Scholar] [CrossRef]
- Anderson, K.; Mutemaringa, T.; Technau, K.G.; Johnson, L.F.; Braithwaite, K.; Mokotoane, E.; Boulle, A.; Davies, M.-A.; on behalf of IeDEA-SA Pediatrics. The next generation: Pregnancy in adolescents and women living with perinatally acquired HIV in South Africa. S. Afr. Med. J. 2021, 111, 260–264. [Google Scholar] [CrossRef]
- Fennell, C.; Seage, G.R.; Zash, R.; Phiri, K.; Diseko, M.; Mayondi, G.; Lockman, S.; Sekoto, T.; Mmalane, M.; Makhema, J.; et al. Adverse Birth Outcomes in Botswana Among Women With Vertically or Horizontally Acquired Human Immunodeficiency Virus. J. Pediatr. Infect. Dis. Soc. 2021, 10, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Jao, J.; Kacanek, D.; Williams, P.L.; Geffner, M.E.; Livingston, E.G.; Sperling, R.S.; Patel, K.; Bardeguez, A.D.; Burchett, S.K.; Chakhtoura, N.; et al. Birth Weight and Preterm Delivery Outcomes of Perinatally vs. Nonperinatally Human Immunodeficiency Virus-Infected Pregnant Women in the United States: Results From the PHACS SMARTT Study and IMPAACT P1025 Protocol. Clin. Infect. Dis. 2017, 65, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Lazenby, G.B.; Mmeje, O.; Fisher, B.M.; Weinberg, A.; Aaron, E.K.; Keating, M.; Luque, A.E.; Willers, D.; Cohan, D.; Money, D. Antiretroviral Resistance and Pregnancy Characteristics of Women with Perinatal and Nonperinatal HIV Infection. Infect. Dis. Obstet. Gynecol. 2016, 2016, 4897501. [Google Scholar] [CrossRef]
- Patel, K.; Karalius, B.; Powis, K.; Kacanek, D.; Berman, C.; Moscicki, A.-B.; Paul, M.; Tassiopoulos, K.; Seage, G.R. Trends in post-partum viral load among women living with perinatal HIV infection in the USA: A prospective cohort study. Lancet HIV 2020, 7, e184–e192. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Evans, C. Children who are HIV-exposed and uninfected: Evidence for action. AIDS 2023, 37, 205–215. [Google Scholar] [CrossRef]
- Jao, J.; Kacanek, D.; Yu, W.; Williams, P.L.; Patel, K.; Burchett, S.; Scott, G.; Abrams, E.J.; Sperling, R.S.; Van Dyke, R.B.; et al. Neurodevelopment of HIV-Exposed Uninfected Infants Born to Women With Perinatally Acquired HIV in the United States. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 84, 213–219. [Google Scholar] [CrossRef]
- Powis, K.M.; Slogrove, A.L.; Okorafor, I.; Millen, L.; Posada, R.; Childs, J.; Abrams, E.J.; Sperling, R.S.; Jao, J. Maternal Perinatal HIV Infection Is Associated With Increased Infectious Morbidity in HIV-exposed Uninfected Infants. Pediatr. Infect. Dis. J. 2019, 38, 500–502. [Google Scholar] [CrossRef]
- Yu, W.; Jacobson, D.L.; Williams, P.L.; Patel, K.; Geffner, M.E.; Van Dyke, R.B.; Kacanek, D.; DiMeglio, L.A.; Jao, J. Growth patterns of uninfected children born to women living with perinatally versus nonperinatally acquired HIV. AIDS 2022, 36, 593–603. [Google Scholar] [CrossRef]
- Muenchhoff, M.; Prendergast, A.J.; Goulder, P.J.R. Immunity to HIV in Early Life. Front. Immunol. 2014, 5, 391. [Google Scholar] [CrossRef]
- Unsal, A.B.; Mattingly, A.S.; Jones, S.E.; Purdy, J.B.; Reynolds, J.C.; Kopp, J.B.; Hazra, R.; Hadigan, C.M. Effect of Antiretroviral Therapy on Bone and Renal Health in Young Adults Infected With HIV in Early Life. J. Clin. Endocrinol. Metab. 2017, 102, 2896–2904. [Google Scholar] [CrossRef]
- Waalewijn, H.; Turkova, A.; Rakhmanina, N.; Cressey, T.R.; Penazzato, M.; Colbers, A.; Burger, D.M. Optimizing Pediatric Dosing Recommendations and Treatment Management of Antiretroviral Drugs Using Therapeutic Drug Monitoring Data in Children Living With HIV. Ther. Drug Monit. 2019, 41, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Grinspoon, S.K.; Fitch, K.V.; Zanni, M.V.; Fichtenbaum, C.J.; Umbleja, T.; Aberg, J.A.; Overton, E.T.; Malvestutto, C.D.; Bloomfield, G.S.; Currier, J.S.; et al. Pitavastatin to Prevent Cardiovascular Disease in HIV Infection. N. Engl. J. Med. 2023, 389, 687–699. [Google Scholar] [CrossRef] [PubMed]
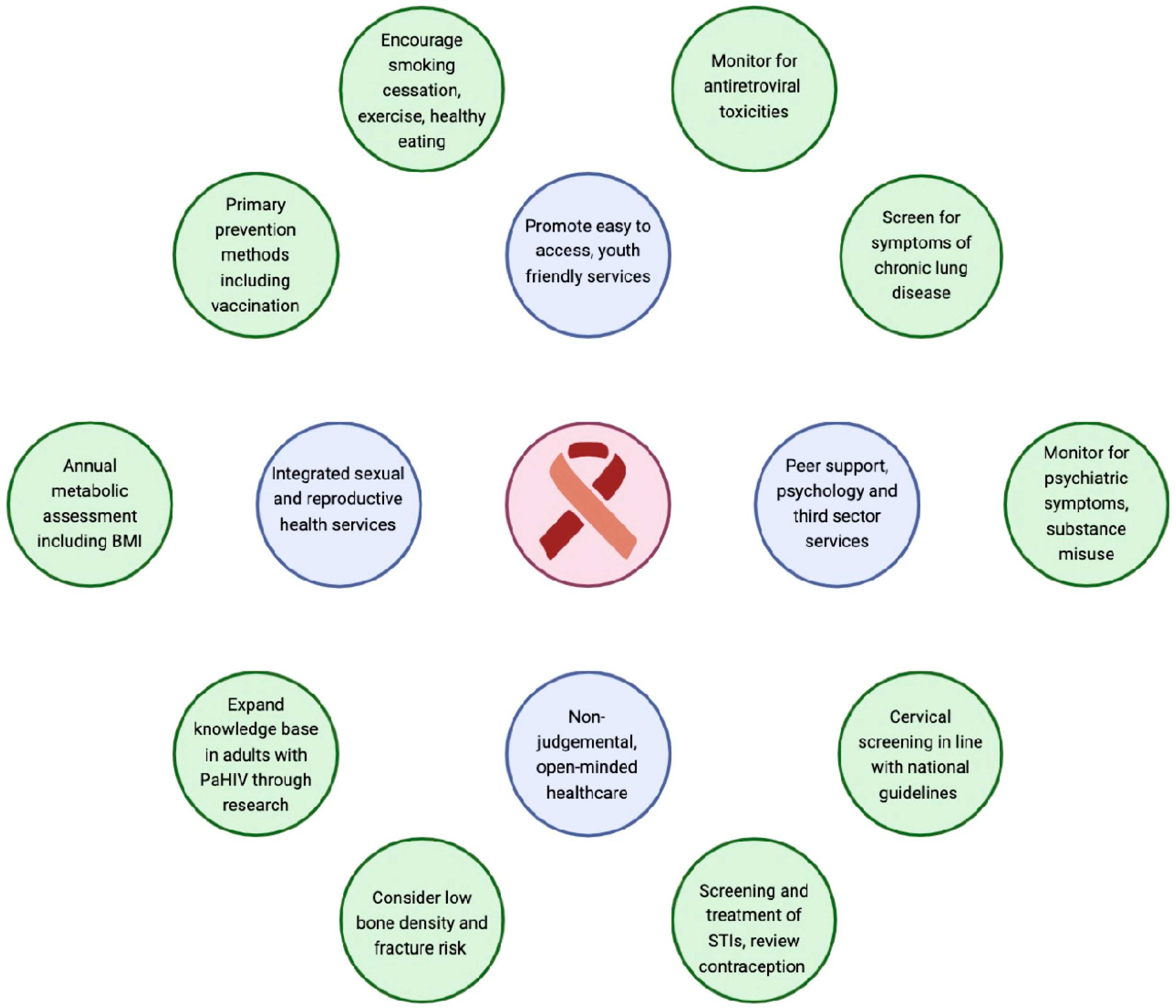
| Monitor | Management | Data Gaps |
|---|---|---|
| Engagement in care | Youth-friendly services removing barriers to accessing care within local service provision | Accurate linkage of paediatric and adult data sets where data are disaggregated by route of transmission (PaHIV versus nPaHIV) How best to re-engage those who have disengaged from care? Effective adherence interventions for young adults, including cost effectiveness? |
| Mortality | Maintaining engagement in care and access to integrated services during transition to adult care. Optimising viral suppression |
| Monitor | Management | Data Gaps |
|---|---|---|
| Viral load | Resistance sequence in virological failure | Emergent incidence of integrase resistance with wide-spread global use of dolutegravir Optimal ART sequencing where integrase resistance occurs Potential for use of long-acting ART in non suppressed youth |
| Adherence | ART optimisation to single tablet regimens where possible |
| Monitor | Management | Data Gaps |
|---|---|---|
| Mental health at each clinic visit | Access to integrated mental health services and peer support | Rates of psychosis in adults LWPaHIV; how does this compare with the general population, HEU young adults and those LWnPaHIV? What are the causes of adverse mental health outcomes and the role of HIV-related factors, such as inflammation versus traditional risk factors? Neurocognitive outcomes in adult life; impact of quality of life and brain aging in 5th decade and beyond |
| Substance use at each clinic visit | Advice and information around substance misuse starting in early adolescence Early referral to cessation services when substance use is identified |
| Monitor | Management | Data Gaps |
|---|---|---|
| Weight/BMI at each clinic visit | If they are overweight, provide lifestyle advice and refer to the dietician Review ART regimen | Long-term risks associated with obesity and chronic inflammation; how does this compare with the general population? What is the role of ART versus traditional risk factors towards CVD? Is there an increased risk of CVD in this group and what is the mechanism? What are the optimal CVD interventions to avoid complications and polypharmacy? |
| Blood pressure at each clinic visit | If there is raised blood pressure, refer to hypertension guidelines for further investigation and management | |
| Metabolic assessment: Annual Hba1c and lipids | If abnormal, provide lifestyle advice, refer to the dietician and where appropriate, provide treatment | |
| Monitor liver function every 6 months | If abnormal, non-invasive liver screening including ultrasound and fibroscan for NAFLD | |
| Selected high-risk groups for enhanced monitoring; e.g., hypertensive, high BMI, high lipids and smokers | Where available, refer to a CVD clinic for enhanced monitoring and intervention | |
| Monitor for alcohol misuse | Refer to support services |
| Monitor | Management | Data Gaps |
|---|---|---|
| Screen for symptoms of CLD—shortness of breath, cough and/or sputum and wheezing [105] | Refer for spirometry. If abnormal, provide referral to respiratory specialist, +/− CT imaging. Assess for concomitant chronic diseases. | Unknown long-term progression of CLD Research towards novel biomarkers to understand mechanisms of CLD |
| Exacerbations of CLD | Sputum cultures (MC + S; TB). Consider prophylactic antibiotics and rescue packs, as per national guidelines for the management of CLD. Annual influenza vaccine. COVID-19/pneumococcal vaccine, as per national guidance | |
| Smoking and/or vaping use | Smoking cessation services | Data to determine the risk of health impacts in people with PaHIV compared to the age-matched HIV-negative population |
| Monitor | Management | Data Gaps |
|---|---|---|
| Fracture risk | Entry to adult care—consider baseline dual-energy X-ray absorptiometry scan in those with additional risk factors such as reduced mobility. If abnormal, refer to osteopenia/osteoporosis guidelines and bone specialist. | Future fracture risk in adults with PaHIV and its association with low BMD, vitamin D and ART |
| Annual blood vitamin D and calcium levels | Replacement if deficient |
| Monitor | Management | Data Gaps |
|---|---|---|
| HIV RNA | SRH education Peer support PrEP for partners if there is a viral rebound | SRH in transgender people with PaHIV STI rates in people with PaHIV Duration of immunity following HPV vaccination and need for booster doses Role of early HPV screening for people with a cervix with PaHIV HPV persistence and complications in young men with PaHIV Developmental outcomes for HEUs born to people LWPaHIV |
| STI/blood-borne virus rates | SRH education Condom use Treatment of STIs Vaccination—HPV/Hepatitis A+B, M Pox | |
| HPV-associated cervical dyplasia and cancers | National cervical screening Referral to colposcopy as per national/local guidelines | |
| Pregnancy surveillance | Referral for specialist HIV antenatal care where available Unintended pregnancies—referral for abortion and contraceptive services | |
| Pregnancy and birth outcomes | Peer support and HIV antenatal care HIV RNA suppression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, M.; Fidler, S.; Foster, C. Adults with Perinatally Acquired HIV; Emerging Clinical Outcomes and Data Gaps. Trop. Med. Infect. Dis. 2024, 9, 74. https://doi.org/10.3390/tropicalmed9040074
Henderson M, Fidler S, Foster C. Adults with Perinatally Acquired HIV; Emerging Clinical Outcomes and Data Gaps. Tropical Medicine and Infectious Disease. 2024; 9(4):74. https://doi.org/10.3390/tropicalmed9040074
Chicago/Turabian StyleHenderson, Merle, Sarah Fidler, and Caroline Foster. 2024. "Adults with Perinatally Acquired HIV; Emerging Clinical Outcomes and Data Gaps" Tropical Medicine and Infectious Disease 9, no. 4: 74. https://doi.org/10.3390/tropicalmed9040074
APA StyleHenderson, M., Fidler, S., & Foster, C. (2024). Adults with Perinatally Acquired HIV; Emerging Clinical Outcomes and Data Gaps. Tropical Medicine and Infectious Disease, 9(4), 74. https://doi.org/10.3390/tropicalmed9040074





