Material Challenges and Hydrogen Embrittlement Assessment for Hydrogen Utilisation in Industrial Scale
Abstract
:1. Introduction
2. Hydrogen Embrittlement
- -
- Hydrogen environmental embrittlement represents the conditions where metals are exposed to a high-pressure, gaseous hydrogen environment.
- -
- Internal hydrogen embrittlement is the degradation of a metal’s mechanical properties during forming or finishing operations, and results in the unintentional introduction of hydrogen into susceptible metals or alloys (e.g., via electrodeposition).
- -
- Hydrogen reaction embrittlement is the degradation of certain mechanical properties when hydrogen reacts with the metal matrix itself to form metallic compounds, such as metal hydride, at relatively low temperatures [22].
3. Testing Methods for Hydrogen Embrittlement
3.1. Mechanical Testing
3.2. Hydrogen Charging during Testing
3.2.1. Pre-Charging at Elevated Temperature/Pressure (Ex Situ)
3.2.2. Hydrogen Charging In Situ
3.2.3. Electrochemical Charging (Ex Situ/In Situ)/Hydrogen Charging in Acid Aqueous Medium (In Situ)
4. ASTM Standards Assessing Hydrogen Embrittlement
4.1. ASTM G142-98 and G129-00
4.2. ASTM F1459-06
4.3. ASTM G142-98
4.4. ASTM G129-00
4.5. ASTM F1624-12
4.6. ASTM E1681-03
4.7. Adapting Standards to Industrial Settings
5. Hydrogen in Industrial Materials
5.1. Hydrogen in Pipeline Steels
5.2. H2 in High-Pressure Hydrogen Storage Tanks
5.3. Hydrogen Embrittlement in Ammonia Processing Systems
5.4. Metal Hydride Formation
5.5. HE in Alloys at Elevated Temperatures
6. Key Areas to Address Hydrogen Embrittlement in Industrial Environment and the Challenges
6.1. Choice of Materials for Engineering Applications
6.2. Choice of Barrier Coatings for Prevention of Hydrogen Permeation
6.3. Choice of Testing Methods for Industrial Applications
6.4. Retrofitting of Plants in Heavy Industries
6.4.1. Iron Ore Pelletising
6.4.2. Alumina Calcination
6.4.3. Clinker Production
7. Conclusions and Outlook
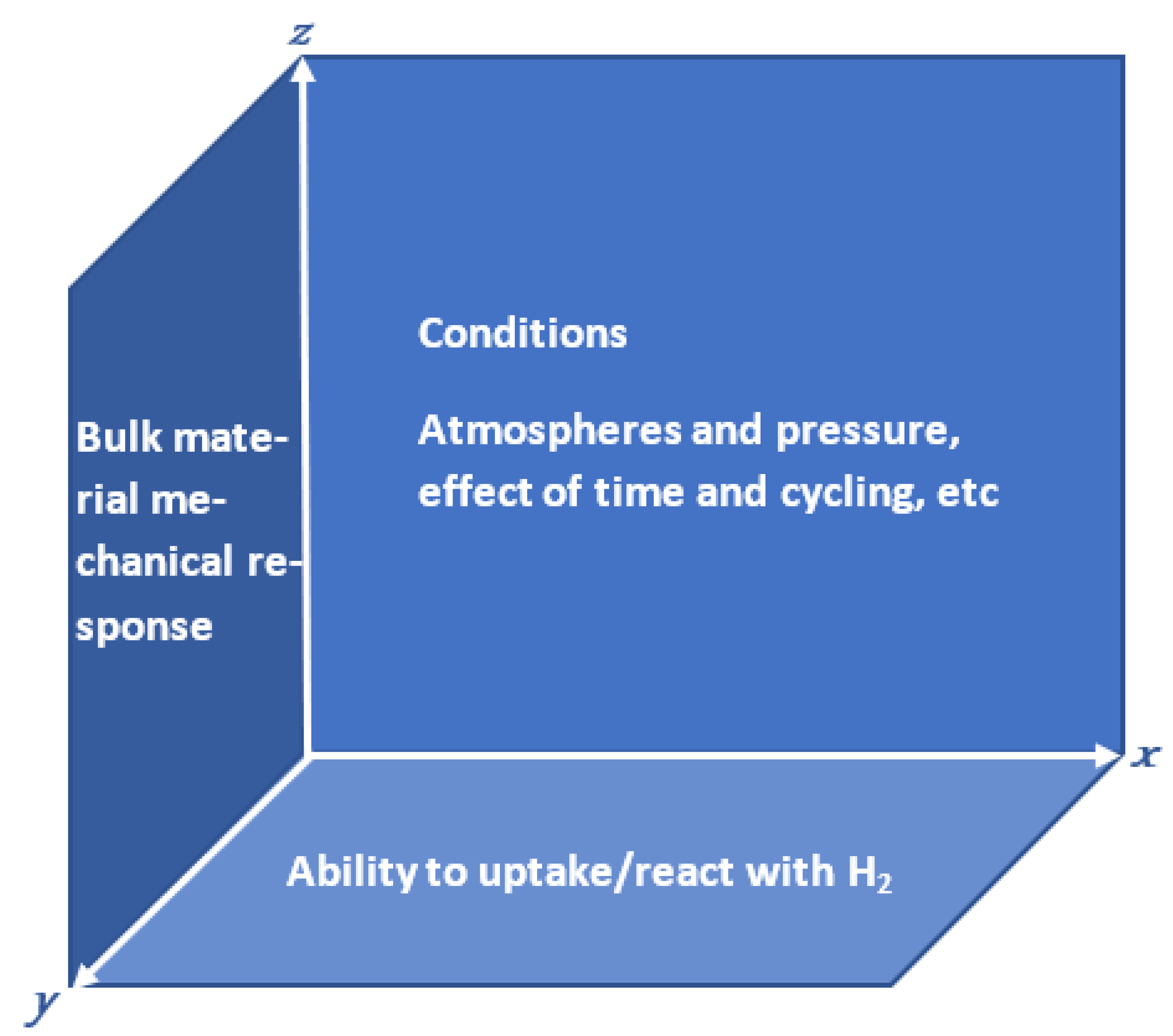
- Limitations of laboratory assessment methods: these are more likely ‘two-dimensional’, trying to link the ability of a material to uptake/react with hydrogen with its intrinsic properties or resulting mechanical (bulk) properties.
- Applicability of ASTM standards for materials testing for industrial use: test conditions differ from industrial tests, and shapes, sizes, and forms are standardised and often differ from those used in industrial processes.
- Limited industrial samples: little data are available for materials exposed to hydrogen-containing atmospheres.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ASTM | American Society for Testing and Materials |
| DPT | disc pressure test |
| FCGR | fatigue-crack growth |
| EAC | environmentally assisted cracking |
| EL | elongation fracture |
| HE | hydrogen embrittlement |
| HGE | hydrogen gas embrittlement |
| KEAC | Stress intensity factor threshold for environmentally assisted cracking |
| KIc | intensity factor for fracture resistance determination |
| LIST | linearly increasing stress test |
| PHe/PH2 | Rupture pressure ratio |
| RA | reduction area |
| SSR | slow strain rate |
| SSRT | slow strain rate tensile |
| UTS | ultimate tensile strength |
References
- Boot, T.; Riemslag, T.; Reinton, E.; Liu, P.; Walters, C.L.; Popovich, V. Assessing the Susceptibility of Existing Pipelines to Hydrogen Embrittlement. In Proceedings of the TMS 2021 150th Annual Meeting & Exhibition, Online, 15–18 March 2021; Springer: Cham, Switzerland, 2021; pp. 722–729. [Google Scholar]
- Li, H.; Niu, R.; Li, W.; Lu, H.; Cairney, J.; Chen, Y.-S. Hydrogen in pipeline steels: Recent advances in characterization and embrittlement mitigation. J. Nat. Gas Sci. Eng. 2022, 105, 104709. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Sijacki Zeravcic, V.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement, 1st ed.; Springer: Singapore, 2016. [Google Scholar]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. B 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Martin, M.L.; Dadfarnia, M.; Nagao, A.; Wang, S.; Sofronis, P. Enumeration of the hydrogen-enhanced localized plasticity mechanism for hydrogen embrittlement in structural materials. Acta Mater. 2019, 165, 734–750. [Google Scholar] [CrossRef]
- Gangloff, R.P. Critical Issues in Hydrogen Assisted Cracking of Structural Alloys. In Environment-Induced Cracking of Materials; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Industrial Components: Prediction, Prevention, and Models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Recent developments in the study of hydrogen embrittlement: Hydrogen effect on dislocation nucleation. Acta Mater. 2010, 58, 5274–5285. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. HYDROGEN IN METALS: Microstructural Aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Katz, Y.; Tymiak, N.; Gerberich, W.W. Nanomechanical probes as new approaches to hydrogen/deformation interaction studies. Eng. Fract. Mech. 2001, 68, 619–646. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Rajicic, B.; Sedmak, A.; Mitrovic, R.; Miskovic, Z. Towards a unified and practical industrial model for prediction of hydrogen embrittlement and damage in steels. Procedia Struct. Integr. 2016, 2, 604–611. [Google Scholar] [CrossRef]
- Chandler, W.T. Hydrogen Environment Embrittlement and Its Control in High Pressure Hydrogen/Oxygen Rocket Engines. In Proceedings of the Advanced Earth-to-Orbit Propulsion Technology, Huntsville, AL, USA, 13–15 May 1986; pp. 618–634. [Google Scholar]
- Toribio, J.; Vergara, D.; Lorenzo, M. Role of in-service stress and strain fields on the hydrogen embrittlement of the pressure vessel constituent materials in a pressurized water reactor. Eng. Fail. Anal. 2017, 82, 458–465. [Google Scholar] [CrossRef]
- Gray, H.R.; Joyce, J.P. Hydrogen Embrittlement of Turbine Disk Alloys. In Proceedings of the Effects of Hydrogen on Behavior of Materials, Moran, WY, USA, 7 September 1975; pp. 578–588. [Google Scholar]
- Neuharth, J.J.; Cavalli, M.N. Investigation of high-temperature hydrogen embrittlement of sensitized austenitic stainless steels. Eng. Fail. Anal. 2015, 49, 49–56. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Liu, C.; Hu, Q.; Zhang, R.; Xu, X.; Yang, H.; Ning, Y.; Li, Y. Study on hydrogen embrittlement susceptibility of X80 steel through in-situ gaseous hydrogen permeation and slow strain rate tensile tests. Int. J. Hydrogen Energy 2023, 48, 243–256. [Google Scholar] [CrossRef]
- Chae, M.J.; Kim, J.H.; Moon, B.; Park, S.; Lee, Y.S. The present condition and outlook for hydrogen-natural gas blending technology. Korean J. Chem. Eng. 2022, 39, 251–262. [Google Scholar] [CrossRef]
- Mahajan, D.; Tan, K.; Venkatesh, T.; Kileti, P.; Clayton, C.R. Hydrogen Blending in Gas Pipeline Networks—A Review. Energies 2022, 15, 3582. [Google Scholar] [CrossRef]
- Johnson, W.H. On some remarkable changes produced in iron and steel by the action of hydrogen and acids. Proc. Proc. R. Soc. Lond. 1875, 23, 168–179. [Google Scholar] [CrossRef]
- Lee, J.A. Hydrogen Embrittlement; National Aeronautics and Space Administration (NASA), ASA Langley Research Center: Hampton, VA, USA, 2016. [Google Scholar]
- Gabetta, G.; Cioffi, P.; Bruschi, R. Engineering thoughts on Hydrogen Embrittlement. Procedia Struct. Integr. 2018, 9, 250–256. [Google Scholar] [CrossRef]
- Del-Pozo, A.; Villalobos, J.C.; Serna, S. 6—A general overview of hydrogen embrittlement. In Current Trends and Future Developments on (Bio-)Membranes; Basile, A., Gallucci, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–168. [Google Scholar]
- Martin, M.L.; Connolly, M.J.; DelRio, F.W.; Slifka, A.J. Hydrogen embrittlement in ferritic steels. Appl. Phys. Rev. 2020, 7, 041301. [Google Scholar] [CrossRef]
- Arniella, V.; Zafra, A.; Álvarez, G.; Belzunce, J.; Rodríguez, C. Comparative study of embrittlement of quenched and tempered steels in hydrogen environments. Int. J. Hydrogen Energy 2022, 47, 17056–17068. [Google Scholar] [CrossRef]
- Michler, T.; Naumann, J. Influence of high pressure hydrogen on the tensile and fatigue properties of a high strength Cu–Al–Ni–Fe alloy. Int. J. Hydrogen Energy 2010, 35, 11373–11377. [Google Scholar] [CrossRef]
- Louthan, M.R. Hydrogen Embrittlement of Metals: A Primer for the Failure Analyst. J. Fail. Anal. Prev. 2008, 8, 289–307. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of Hydrogen Embrittlement in Metals: Hydrogen Diffusion, Hydrogen Characterization, Hydrogen Embrittlement Mechanism and Prevention. Acta Metall. Sin. Engl. Lett. 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Khalid, H.; Mansoor, B. Hydrogen Embrittlement in Nickel-Base Superalloy 718. In Recent Developments in Analytical Techniques for Corrosion Research; Toor, I.U., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 279–306. [Google Scholar]
- Marchi, C.S. Chapter 3.7 Austenitic Stainless Steels. In Gaseous Hydrogen Embrittlement of High Performance Metals in Energy Systems; Gangloff, R.P., Somerday, B.P., Eds.; Sandia National Laboratories: Livermore, CA, USA, 2011. [Google Scholar]
- Koren, E.; Hagen, C.M.H.; Wang, D.; Lu, X.; Johnsen, R.; Yamabe, J. Experimental comparison of gaseous and electrochemical hydrogen charging in X65 pipeline steel using the permeation technique. Corros. Sci. 2023, 215, 111025. [Google Scholar] [CrossRef]
- Kim, J.; Tasan, C.C. Microstructural and micro-mechanical characterization during hydrogen charging: An In Situ scanning electron microscopy study. Int. J. Hydrogen Energy 2019, 44, 6333–6343. [Google Scholar] [CrossRef]
- Lee, D.-H.; Jung, J.Y.; Lee, K.H.; Lee, S.Y.; Zhao, Y.; Lau, K.B.; Wang, P.; Ramamurty, U. Distinct effects of in-situ and ex-situ hydrogen charging methods on the mechanical behavior of CoCrFeNi high-entropy alloy fabricated by laser-powder bed fusion. J. Alloys Compd. 2023, 940, 168858. [Google Scholar] [CrossRef]
- An, T.; Zheng, S.; Peng, H.; Wen, X.; Chen, L.; Zhang, L. Synergistic action of hydrogen and stress concentration on the fatigue properties of X80 pipeline steel. Mater. Sci. Eng. A 2017, 700, 321–330. [Google Scholar] [CrossRef]
- Atrens, A.; Brosnan, C.C.; Ramamurthy, S.; Oehlert, A.; Smith, I.O. Linearly increasing stress test (LIST) for SCC research. Meas. Sci. Technol. 1993, 4, 1281. [Google Scholar] [CrossRef]
- Nibur, K.A.; Somerday, B.P. 7—Fracture and fatigue test methods in hydrogen gas. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Gangloff, R.P., Somerday, B.P., Eds.; Woodhead Publishing: Sawston, UK, 2012; Volume 2, pp. 195–236. [Google Scholar]
- Ilyushechkin, A.Y.; Carter, L.; Schoeman, L. Characterization of materials exposed to H2-containing atmospheres at elevated temperatures and pressure. In Proceedings of the the International Symposium on Metal-Hydrogen Systems (MH2022), Perth, Australia, 30 October–4 November 2022. [Google Scholar]
- Cheaitani, M.J.; Pargeter, R.J. Fracture Mechanics Techniques for Assessing the Effects of Hydrogen on Steel Properties. In Proceedings of the The International Steel and Hydrogen Conference, Albuquerque, NM, USA, 27–30 September 2011. [Google Scholar]
- Bilotta, G.; Henaff, G.; Halm, D.; Arzaghi, M. Experimental measurement of out-of-plane displacement in crack propagation under gaseous hydrogen. Int. J. Hydrogen Energy 2017, 42, 10568–10578. [Google Scholar] [CrossRef]
- ASTM G142-98; Standard Test Method for Determination of Susceptibility of Metals to Embrittlement in Hydrogen Containing Environments at High Pressure, High Temperature, or Both. ASTM International: West Conshohocken, PA, USA, 2000; pp. 1–8. [CrossRef]
- ASTM G129-00; Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking. ASTM International: West Conshohocken, PA, USA, 2000; pp. 1–7. [CrossRef]
- ASTM F1459-06; Standard Test Method for Determination of the Susceptibility of Metallic Materials to Hydrogen Gas Embrittlement (HGE). ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–3. [CrossRef]
- ASTM F1624-12; Standard Test Method for Measurement of Hydrogen Embrittlement Threshold in Steel by the Incremental Step Loading Technique. ASTM International: West Conshohocken, PA, USA, 2012; pp. 1–12. [CrossRef]
- ASTM E1681-03; Standard Test Method for Determining Threshold Stress Intensity Factor for Environment-Assisted Cracking of Metallic Materials. ASTM International: West Conshohocken, PA, USA,, 2008; pp. 1–13. [CrossRef]
- Lee, J.A. Hydrogen embrittlement of nickel, cobalt and iron-based superalloys. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing: Sawston, UK, 2012; pp. 624–667. [Google Scholar]
- Fidelle, J.P.; Allemand, L.R.; Roux, C.; Rapin, M. In Proceedings of the Hydrogen in Metals, French, 1967; pp. 131–172.
- Fidelle, J.P.; Broudeur, R.; Pirrovani, C.; Roux, C. Disk pressure technique. In Proceedings of the Symposium on Hydrogn Embrittlement Testing—ASTM STP 543, Los Angeles, CA, USA, June 1974. [Google Scholar]
- Zhou, D.H.; Zhou, W.X.; Xu, Z.L. Hydrogen degradation of 21-6-9 and medium carbon steel by disc pressure test. J. Nucl. Mater. 1986, 141–143, 503–507. [Google Scholar] [CrossRef]
- Leunis, E.; Duprez, L. Selecting hydrogen embrittlement resistant materials by means of the disc rupture test. In Proceedings of the 18th World Hydrogen Energy Conference 2010 (WHEC 2010), Essen, Germany, 16–21 May 2010; pp. 289–294. [Google Scholar]
- Beghini, M.; Benamati, G.; Bertini, L. Hydrogen Embrittlement Characterization by Disk Pressure Tests: Test Analysis and Application to High Chromium Martensitic Steels. J. Eng. Mater. Technol. 1996, 118, 179–185. [Google Scholar] [CrossRef]
- Charles, Y.; Gaspérini, M.; Disashi, J.; Jouinot, P. Numerical modeling of the Disk Pressure Test up to failure under gaseous hydrogen. J. Mater. Process. Technol. 2012, 212, 1761–1770. [Google Scholar] [CrossRef]
- ASTM E399-17; Standard Test Method for Linear-Elastic Plane-Strain Fracture Toughness of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2019; pp. 1–34. [CrossRef]
- ASTM E740/E740M-03; Standard Practice for Fracture Testing with Surface-Crack Tension Specimens. ASTM International: West Conshohocken, PA, USA, 2016; pp. 1–9. [CrossRef]
- ASTM E1820-18; Standard Test Method for Measurement of Fracture Toughness. ASTM International: West Conshohocken, PA, USA, 2019; pp. 1–55. [CrossRef]
- ASTM E2899-19e1; Standard Test Method for Measurement of Initiation Toughness in Surface Cracks Under Tension and Bending. ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–40. [CrossRef]
- Briottet, L.; Batisse, R.; Bernard, P.; Duret-Thual, C.; Heuzé, J.-L.; Martin, F.; Thebault, F.; Vucko, F. 10—Industrial Consequences of Hydrogen Embrittlement. In Mechanics-Microstructure-Corrosion Coupling; Blanc, C., Aubert, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–244. [Google Scholar]
- Trasatti, S.P.; Sivieri, E.; Mazza, F. Susceptibility of a X80 steel to hydrogen embrittlement. Mater. Corros. 2005, 56, 111–117. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Komoda, R.; Yamada, K.; Kubota, M.; Ginet, P.; Barbier, F.; Furtado, J.; Prost, L. The inhibitory effect of carbon monoxide contained in hydrogen gas environment on hydrogen-accelerated fatigue crack growth and its loading frequency dependency. Int. J. Hydrogen Energy 2019, 44, 29007–29016. [Google Scholar] [CrossRef]
- Komoda, R.; Kubota, M.; Staykov, A.; Ginet, P.; Furtado, J.; Prost, L.; Nagao, A. Loading Dependence of Mitigation Effect of CO on Hydrogen Embrittlement of Pure Iron and Low Carbon Steel. In Proceedings of the 32nd International Ocean and Polar Engineering Conference, Shanghai, China, 6–10 June 2022. [Google Scholar]
- Liu, C.; Yang, H.; Wang, C.; Zhang, H.; Ding, R.; Ai, L.; Fan, X.; Zhang, R.; Xu, X.; Ning, Y.; et al. Effects of CH4 and CO on hydrogen embrittlement susceptibility of X80 pipeline steel in hydrogen blended natural gas. Int. J. Hydrogen Energy 2023, 48, 27766–27777. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, B.; Wang, J.; Hu, S.; Ye, B.; He, Y.; Zheng, J.; Zhang, L. Effect of interaction between corrosion film and H2S/CO2 partial pressure ratio on the hydrogen permeation in X80 pipeline steel. Corros. Eng. Sci. Technol. 2020, 55, 392–399. [Google Scholar] [CrossRef]
- Somerday, B. Hydrogen Embrittlement of Structural Steels; DOE Hydrogen and Fuel Cells Program: Washington, DC, USA, 2013; pp. III-29–III-32. [Google Scholar]
- Onyango, D. Britain’s Gas Grid to Accept 20% Hydrogen Blend by 2023, Preparations Underway. Pipeline Technol. J. 2022. [Google Scholar]
- Boot, T.; Riemslag, T.A.C.; Reinton, E.T.E.; Liu, P.; Walters, C.L.; Popovich, V. In-situ hollow sample setup design for mechanical characterisation of gaseous hydrogen embrittlement of pipeline steels and welds. Metals 2021, 11, 1242. [Google Scholar] [CrossRef]
- Laureys, A.; Depraetere, R.; Cauwels, M.; Depover, T.; Hertelé, S.; Verbeken, K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. J. Nat. Gas Sci. Eng. 2022, 101, 104534. [Google Scholar] [CrossRef]
- Erdener, B.C.; Sergi, B.; Guerra, O.J.; Lazaro Chueca, A.; Pambour, K.; Brancucci, C.; Hodge, B.-M. A review of technical and regulatory limits for hydrogen blending in natural gas pipelines. Int. J. Hydrogen Energy 2023, 48, 5595–5617. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. HyBlend Project to Accelerate Potential for Blending Hydrogen in Natural Gas Pipelines. Available online: https://www.nrel.gov/news/program/2020/hyblend-project-to-accelerate-potential-for-blending-hydrogen-in-natural-gas-pipelines.html (accessed on 5 February 2023).
- Ogden, J.; Jaffe, A.M.; Scheitrum, D.; McDonald, Z.; Miller, M. Natural gas as a bridge to hydrogen transportation fuel: Insights from the literature. Energy Policy 2018, 115, 317–329. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Furtado, J.; Barbier, F. Hydrogen Embrittlement-Related Issues and Needs in the Hydrogen Value Chain. In Proceedings of the International Hydrogen Conference (IHC 2012): Hydrogen-Materials Interactions, Moran, WY, USA, 9–12 September 2012; Somerday, B.P., Sofronis, P., Eds.; ASME Press: New York, NY, USA, 2014. [Google Scholar]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Barhoumi, E.M.; Ben Belgacem, I.; Mansir, I.B.; Aliyu, M.; Emori, W.; Uzoma, P.C.; Beitelmal, W.H.; Akyüz, E.; Radwan, A.B.; et al. A focused review of the hydrogen storage tank embrittlement mechanism process. Int. J. Hydrogen Energy 2023, 48, 12935–12948. [Google Scholar] [CrossRef]
- Nelson, H.G.; Williams, D.P. Quantitative Observations of Hydrogen-Induced Slow Crack Growth in a Low Alloy Steel; NASA: Washington, DC, USA, 1973; p. 253. [Google Scholar]
- Hua, Z.; Zhang, X.; Zheng, J.; Gu, C.; Cui, T.; Zhao, Y.; Peng, W. Hydrogen-enhanced fatigue life analysis of Cr–Mo steel high-pressure vessels. Int. J. Hydrogen Energy 2017, 42, 12005–12014. [Google Scholar] [CrossRef]
- Matsunaga, H.; Yoshikawa, M.; Kondo, R.; Yamabe, J.; Matsuoka, S. Slow strain rate tensile and fatigue properties of Cr–Mo and carbon steels in a 115 MPa hydrogen gas atmosphere. Int. J. Hydrogen Energy 2015, 40, 5739–5748. [Google Scholar] [CrossRef]
- Jia, G.; Lei, M.; Li, M.; Xu, W.; Li, R.; Lu, Y.; Cai, M. Hydrogen embrittlement in hydrogen-blended natural gas transportation systems: A review. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Lee, S.-I.; Lee, J.-M.; Lee, S.-Y.; Kim, H.-J.; Suh, J.-Y.; Shim, J.-H.; Baek, U.-B.; Nahm, S.-H.; Lee, J.; Hwang, B. Tensile and fracture behaviors of austenitic high-manganese steels subject to different hydrogen embrittlement test methods. Mater. Sci. Eng. A 2019, 766, 138367. [Google Scholar] [CrossRef]
- San Marchi, C.; Somerday, B.P.; Tang, X.; Schiroky, G.H. Effects of alloy composition and strain hardening on tensile fracture of hydrogen-precharged type 316 stainless steels. Int. J. Hydrogen Energy 2008, 33, 889–904. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Michler, T.; Berreth, K.; Naumann, J.; Sattler, E. Analysis of martensitic transformation in 304 type stainless steels tensile tested in high pressure hydrogen atmosphere by means of XRD and magnetic induction. Int. J. Hydrogen Energy 2012, 37, 3567–3572. [Google Scholar] [CrossRef]
- Cui, H.; Wang, W.; Li, A.; Li, M.; Xu, S.; Liu, H. Failure analysis of the brittle fracture of a thick-walled 20 steel pipe in an ammonia synthesis unit. Eng. Fail. Anal. 2010, 17, 1359–1376. [Google Scholar] [CrossRef]
- Kain, V.; Gupta, V.; De, P.K. Embrittlement cracking of a stabilized stainless steel wire mesh in an ammonia converter. In Environment-Induced Cracking of Materials; Shipilov, S.A., Jones, R.H., Olive, J.M., Rebak, R.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 411–420. [Google Scholar]
- Young, K. Metal Hydrides. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Abdin, Z.; Webb, C.J.; Gray, E.M. One-dimensional metal-hydride tank model and simulation in Matlab–Simulink. Int. J. Hydrogen Energy 2018, 43, 5048–5067. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Afzal, M.; Mane, R.; Sharma, P. Heat transfer techniques in metal hydride hydrogen storage: A review. Int. J. Hydrogen Energy 2017, 42, 30661–30682. [Google Scholar] [CrossRef]
- Bellosta von Colbe, J.; Ares, J.-R.; Barale, J.; Baricco, M.; Buckley, C.; Capurso, G.; Gallandat, N.; Grant, D.M.; Guzik, M.N.; Jacob, I.; et al. Application of hydrides in hydrogen storage and compression: Achievements, outlook and perspectives. Int. J. Hydrogen Energy 2019, 44, 7780–7808. [Google Scholar] [CrossRef]
- Symons, D.M. A Comparison of Internal Hydrogen Embrittlement and Hydrogen Environment Embrittlement of X-750; Bettis Atomic Power Laboratory, Bechtel Bettis Inc: West Mifflin, PA, USA, 1999. [Google Scholar]
- Clark, W.G. Effect of temperature and pressure on hydrogen cracking in high strength type 4340 steel. J. Mater. Energy Syst. 1979, 1, 33–40. [Google Scholar] [CrossRef]
- Fukuyama, S.; Yokogawa, K. Effect of heat treatments on hydrogen environment embrittlement of alloy 718. Superalloys 1994, 718, 625–706. [Google Scholar]
- Galliano, F.; Andrieu, E.; Cloué, J.-M.; Odemer, G.; Blanc, C. Effect of temperature on hydrogen embrittlement susceptibility of alloy 718 in Light Water Reactor environment. Int. J. Hydrogen Energy 2017, 42, 21371–21378. [Google Scholar] [CrossRef]
- Chêne, J.; Brass, A.M. Role of temperature and strain rate on the hydrogen-induced intergranular rupture in alloy 600. Metall. Mater. Trans. A 2004, 35, 457–464. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Qin, M.; Li, Q.; Zhang, Z.; Chen, K.; Li, Y.; Hu, H.; Wu, W.; Zhang, J. Effect of high temperature deformation on the microstructure, mechanical properties and hydrogen embrittlement of 2.25Cr–1Mo-0.25 V steel. Int. J. Hydrogen Energy 2017, 42, 24549–24559. [Google Scholar] [CrossRef]
- Wetegrove, M.; Duarte, M.J.; Taube, K.; Rohloff, M.; Gopalan, H.; Scheu, C.; Dehm, G.; Kruth, A. Preventing Hydrogen Embrittlement: The Role of Barrier Coatings for the Hydrogen Economy. Hydrogen 2023, 4, 307–322. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Yani, I.; Mataram, A.; Gunawan; Basri, H. The Effect of the Welding Direction on Fatigue Crack Propagation Rate of Welded Shell Kiln. J. Phys. Conf. Ser. 2019, 1198, 042013. [Google Scholar] [CrossRef]
- ASTM A370; Standard Test Methods and Definitions for Mechanical Testing of Steel Product. ASTM International: West Conshohocken, PA, USA, 2022.
- Mineral Products Association; Cinar Ltd.; VDZ gGmbH. Options for Switching UK Cement Production Sites to Near Zero CO2 Emission Fuel: Technical and Financial Feasibility; Mineral Products Association, Cinar Ltd. & VDZ gGmbH, 2019; pp. 1–50. [Google Scholar]
- Hemrick, J.G. Refractory Issues Related to the Use of Hydrogen as An Alternative Fuel. Am. Ceram. Soc. Bull. 2022, 101, 26–31. [Google Scholar]




| Test | Conditions | Parameters |
|---|---|---|
| ASTM G142-98: Standard test method for determination of susceptibility of metals to embrittlement in hydrogen-containing environments at high pressure, high temperature, or both [41] | In H2/air, high temperature, high pressure | Load-displacement curve Plastic elongation Ultimate tensile strength Notched tensile strength |
| ASTM G129-00: Standard practice for slow strain rate testing to evaluate the susceptibility of metallic materials to environmentally assisted cracking [42] | Any with H2 versus control environment | Applying different extension rates: monitoring applied load and crosshead displacement, recorded corrosion potential |
| ASTM F1459-06: Standard test method for determination of the susceptibility of metallic materials to hydrogen gas embrittlement (HGE) [43] | In H2/He, room temperature, high pressure | PHe/PH2 |
| ASTM F1624-12: Standard test method for measurement of hydrogen embrittlement threshold in steel by the incremental step loading technique [44] | In air or controlled environment | Threshold stress by four-point bending |
| ASTM E1681-03: Standard test method for determining the threshold stress intensity factor for environment-assisted cracking of metallic materials [45] | Controlled environment | Stress intensity factor threshold for environmentally assisted cracking, KEAC |
| Cylinder | Liner | Pmax, MPa | |
|---|---|---|---|
| Type I | metal | metal | 20–30 |
| Type II | Metal with wrapped grass/carbon fibre | metal | Not limited |
| Type III | fibre resin composite | metal | 45 |
| Type IV | fibre resin composite | polymer | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyushechkin, A.; Schoeman, L.; Carter, L.; Hla, S.S. Material Challenges and Hydrogen Embrittlement Assessment for Hydrogen Utilisation in Industrial Scale. Hydrogen 2023, 4, 599-619. https://doi.org/10.3390/hydrogen4030039
Ilyushechkin A, Schoeman L, Carter L, Hla SS. Material Challenges and Hydrogen Embrittlement Assessment for Hydrogen Utilisation in Industrial Scale. Hydrogen. 2023; 4(3):599-619. https://doi.org/10.3390/hydrogen4030039
Chicago/Turabian StyleIlyushechkin, Alexander, Liezl Schoeman, Lachlan Carter, and San Shwe Hla. 2023. "Material Challenges and Hydrogen Embrittlement Assessment for Hydrogen Utilisation in Industrial Scale" Hydrogen 4, no. 3: 599-619. https://doi.org/10.3390/hydrogen4030039
APA StyleIlyushechkin, A., Schoeman, L., Carter, L., & Hla, S. S. (2023). Material Challenges and Hydrogen Embrittlement Assessment for Hydrogen Utilisation in Industrial Scale. Hydrogen, 4(3), 599-619. https://doi.org/10.3390/hydrogen4030039







