The Neural Correlates of Developmental Prosopagnosia: Twenty-Five Years on
Abstract
:1. Introduction
2. Literature Search
3. The (In)visible Brain Markers of Developmental Prosopagnosia
3.1. Gray and White Matter Alterations
3.2. Face-Induced Brain Activity in Developmental Prosopagnosia
4. EEG and MEG
5. Literature Weakness
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DP | Developmental prosopagnosia |
| Sample | |
| HCs | Healthy control individuals |
| DPs | Individuals with developmental prosopagnosia |
| Neuroimage technique | |
| (f)MRI | (Functional) Magnetic Resonance Imaging |
| EEG | Electroencephalography |
| MEG | Magnetoencephalography |
| ERP | Event-related potential |
| Brain region: | |
| VOTC | Ventral occipito-temporal cortex |
| FG | Fusiform gyrus |
| FFA | Fusiform face area |
| OFA | Occipital face area |
| ITG | Inferior temporal gyrus |
| MTG | Middle temporal gyrus |
| STS | Superior temporal sulcus |
| DLPFC | Dorso-lateral prefrontal cortex |
| LOC | Lateral occipital cortex |
| TC | Temporal cortex |
| p | posterior |
| a | anterior |
| r- | right |
| l- | left |
References
- Tsao, D.Y.; Livingstone, M.S. Mechanisms of Face Perception. Annu. Rev. Neurosci. 2008, 31, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.; Johnson, M.H. CONSPEC and CONLERN: A Two-Process Theory of Infant Face Recognition. Psychol. Rev. 1991, 98, 164–181. [Google Scholar] [CrossRef] [PubMed]
- McKone, E.; Kanwisher, N.; Duchaine, B.C. Can Generic Expertise Explain Special Processing for Faces? Trends Cogn. Sci. 2007, 11, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Richler, J.J.; Wong, Y.K.; Gauthier, I. Perceptual Expertise as a Shift from Strategic Interference to Automatic Holistic Processing. Curr. Dir. Psychol. Sci. 2011, 20, 129–134. [Google Scholar] [CrossRef]
- Malatesta, G.; Manippa, V.; Tommasi, L. Crying the Blues: The Configural Processing of Infant Face Emotions and Its Association with Postural Biases. Atten. Percept. Psychophys. 2022, 84, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B. Picture-Plane Inversion Leads to Qualitative Changes of Face Perception. Acta Psychol. 2008, 128, 274–289. [Google Scholar] [CrossRef]
- Negrini, M.; Brkić, D.; Pizzamiglio, S.; Premoli, I.; Rivolta, D. Neurophysiological Correlates of Featural and Spacing Processing for Face and Non-Face Stimuli. Front. Psychol. 2017, 8, 333. [Google Scholar] [CrossRef]
- Ventura, M.; Palmisano, A.; Innamorato, F.; Tedesco, G.; Manippa, V.; Caffò, A.O.; Rivolta, D. Face Memory and Facial Expression Recognition Are Both Affected by Wearing Disposable Surgical Face Masks. Cogn. Process 2023, 24, 43–57. [Google Scholar] [CrossRef]
- Ventura, M.; Innamorato, F.; Palmisano, A.; Cicinelli, G.; Nobile, E.; Manippa, V.; Keller, R.; Rivolta, D. Investigating the Impact of Disposable Surgical Face-Masks on Face Identity and Emotion Recognition in Adults with Autism Spectrum Disorder. Autism Res. 2023, 16, 1063–1077. [Google Scholar] [CrossRef]
- Bodamer, J. Die Prosop-Agnosie: Die Agnosie Des Physiognomieerkennens. Arch. Für Psychiatr. Nervenkrankh. 1947, 179, 6–53. [Google Scholar] [CrossRef]
- Damasio, A.R.; Damasio, H.; Van Hoesen, G.W. Prosopagnosia: Anatomic Basis and Behavioral Mechanisms. Neurology 1982, 32, 331. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B. Distinguishing the Cause and Consequence of Face Inversion: The Perceptual Field Hypothesis. Acta Psychol. 2009, 132, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, D.; Lawson, R.P.; Palermo, R. More than Just a Problem with Faces: Altered Body Perception in a Group of Congenital Prosopagnosics. Q. J. Exp. Psychol. 2017, 70, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B.C.; Nakayama, K. Developmental Prosopagnosia: A Window to Content-Specific Face Processing. Curr. Opin. Neurobiol. 2006, 16, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Monti, C.; Sozzi, M.; Bossi, F.; Corbo, M.; Rivolta, D. Atypical Holistic Processing of Facial Identity and Expression in a Case of Acquired Prosopagnosia. Cogn. Neuropsychol. 2019, 36, 358–382. [Google Scholar] [CrossRef]
- Susilo, T.; Duchaine, B. Advances in Developmental Prosopagnosia Research. Curr. Opin. Neurobiol. 2013, 23, 423–429. [Google Scholar] [CrossRef]
- Grüter, T.; Grüter, M.; Carbon, C.-C. Neural and Genetic Foundations of Face Recognition and Prosopagnosia. J. Neuropsychol. 2008, 2, 79–97. [Google Scholar] [CrossRef]
- Perrett, D.I.; Rolls, E.T.; Caan, W. Visual Neurones Responsive to Faces in the Monkey Temporal Cortex. Exp. Brain Res. 1982, 47, 329–342. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef]
- Haxby, J.V.; Gobbini, M.I. Distributed Neural Systems for Face Perception. In The Oxford Handbook of Face Perception; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. The Distributed Human Neural System for Face Perception. Trends Cogn. Sci. 2000, 4, 223–233. [Google Scholar] [CrossRef]
- Meyers, E.M.; Borzello, M.; Freiwald, W.A.; Tsao, D. Intelligent Information Loss: The Coding of Facial Identity, Head Pose, and Non-Face Information in the Macaque Face Patch System. J. Neurosci. 2015, 35, 7069–7081. [Google Scholar] [CrossRef]
- Pitcher, D.; Walsh, V.; Duchaine, B. The Role of the Occipital Face Area in the Cortical Face Perception Network. Exp. Brain Res. 2011, 209, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.; Yovel, G. Two Neural Pathways of Face Processing: A Critical Evaluation of Current Models. Neurosci. Biobehav. Rev. 2015, 55, 536–546. [Google Scholar] [CrossRef]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. Human Neural Systems for Face Recognition and Social Communication. Biol. Psychiatry 2002, 51, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, D.; Walsh, V.; Yovel, G.; Duchaine, B. TMS Evidence for the Involvement of the Right Occipital Face Area in Early Face Processing. Curr. Biol. 2007, 17, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.J.; Ewbank, M.P. Distinct Representations for Facial Identity and Changeable Aspects of Faces in the Human Temporal Lobe. Neuroimage 2004, 23, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Said, C.P.; Moore, C.D.; Engell, A.D.; Todorov, A.; Haxby, J.V. Distributed Representations of Dynamic Facial Expressions in the Superior Temporal Sulcus. J. Vis. 2010, 10, 11. [Google Scholar] [CrossRef]
- Yovel, G.; O’Toole, A.J. Recognizing People in Motion. Trends Cogn. Sci. 2016, 20, 383–395. [Google Scholar] [CrossRef]
- Mazard, A.; Schiltz, C.; Rossion, B. Recovery from Adaptation to Facial Identity Is Larger for Upright than Inverted Faces in the Human Occipito-Temporal Cortex. Neuropsychologia 2006, 44, 912–922. [Google Scholar] [CrossRef]
- Gobbini, M.I.; Haxby, J.V. Neural Systems for Recognition of Familiar Faces. Neuropsychologia 2007, 45, 32–41. [Google Scholar] [CrossRef]
- Leveroni, C.L.; Seidenberg, M.; Mayer, A.R.; Mead, L.A.; Binder, J.R.; Rao, S.M. Neural Systems Underlying the Recognition of Familiar and Newly Learned Faces. J. Neurosci. 2000, 20, 878–886. [Google Scholar] [CrossRef]
- Borghesani, V.; Narvid, J.; Battistella, G.; Shwe, W.; Watson, C.; Binney, R.J.; Sturm, V.; Miller, Z.; Mandelli, M.L.; Miller, B. “Looks Familiar, but I Do Not Know Who She Is”: The Role of the Anterior Right Temporal Lobe in Famous Face Recognition. Cortex 2019, 115, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. Face Familiarity Feelings, the Right Temporal Lobe and the Possible Underlying Neural Mechanisms. Brain Res. Rev. 2007, 56, 214–235. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. Different Patterns of Famous People Recognition Disorders in Patients with Right and Left Anterior Temporal Lesions: A Systematic Review. Neuropsychologia 2007, 45, 1591–1607. [Google Scholar] [CrossRef]
- Di Russo, F.; Berchicci, M.; Bianco, V.; Perri, R.L.; Pitzalis, S.; Quinzi, F.; Spinelli, D. Normative Event-Related Potentials from Sensory and Cognitive Tasks Reveal Occipital and Frontal Activities Prior and Following Visual Events. Neuroimage 2019, 196, 173–187. [Google Scholar] [CrossRef]
- Bentin, S.; Allison, T.; Puce, A.; Perez, E.; McCarthy, G. Electrophysiological Studies of Face Perception in Humans. J. Cogn. Neurosci. 1996, 8, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Gosling, A.; Eimer, M. An Event-Related Brain Potential Study of Explicit Face Recognition. Neuropsychologia 2011, 49, 2736–2745. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Negrini, M.; Nitsche, M.A.; Rivolta, D. Anodal-tDCS over the Human Right Occipital Cortex Enhances the Perception and Memory of Both Faces and Objects. Neuropsychologia 2016, 81, 238–244. [Google Scholar] [CrossRef]
- Towler, J.; Eimer, M. Electrophysiological Studies of Face Processing in Developmental Prosopagnosia: Neuropsychological and Neurodevelopmental Perspectives. Cogn. Neuropsychol. 2012, 29, 503–529. [Google Scholar] [CrossRef]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual Intake of Dietary Flavonoids and Risk of Parkinson Disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef]
- Watanabe, S.; Miki, K.; Kakigi, R. Mechanisms of Face Perception in Humans: A Magneto-and Electro-Encephalographic Study. Neuropathology 2005, 25, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Borra, D.; Bossi, F.; Rivolta, D.; Magosso, E. Deep Learning Applied to EEG Source-Data Reveals Both Ventral and Dorsal Visual Stream Involvement in Holistic Processing of Social Stimuli. Sci. Rep. 2023, 13, 7365. [Google Scholar] [CrossRef] [PubMed]
- Rivolta, D.; Castellanos, N.P.; Stawowsky, C.; Helbling, S.; Wibral, M.; Grützner, C.; Koethe, D.; Birkner, K.; Kranaster, L.; Enning, F. Source-Reconstruction of Event-Related Fields Reveals Hyperfunction and Hypofunction of Cortical Circuits in Antipsychotic-Naive, First-Episode Schizophrenia Patients during Mooney Face Processing. J. Neurosci. 2014, 34, 5909–5917. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J. Non-Spatial Attentional Effects on P1. Clin. Neurophysiol. 2002, 113, 1903–1908. [Google Scholar] [CrossRef]
- Itier, R.J.; Taylor, M.J. Face Recognition Memory and Configural Processing: A Developmental ERP Study Using Upright, Inverted, and Contrast-Reversed Faces. J. Cogn. Neurosci. 2004, 16, 487–502. [Google Scholar] [CrossRef]
- Kaufmann, J.M.; Schweinberger, S.R.; Burton, A.M. N250 ERP Correlates of the Acquisition of Face Representations across Different Images. J. Cogn. Neurosci. 2009, 21, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.W.; Curran, T.; Porterfield, A.L.; Collins, D. Activation of Preexisting and Acquired Face Representations: The N250 Event-Related Potential as an Index of Face Familiarity. J. Cogn. Neurosci. 2006, 18, 1488–1497. [Google Scholar] [CrossRef]
- Eimer, M. Event-Related Brain Potentials Distinguish Processing Stages Involved in Face Perception and Recognition. Clin. Neurophysiol. 2000, 111, 694–705. [Google Scholar] [CrossRef]
- Hasson, U.; Avidan, G.; Deouell, L.Y.; Bentin, S.; Malach, R. Face-Selective Activation in a Congenital Prosopagnosic Subject. J. Cogn. Neurosci. 2003, 15, 419–431. [Google Scholar] [CrossRef]
- Avidan, G.; Hasson, U.; Malach, R.; Behrmann, M. Detailed Exploration of Face-Related Processing in Congenital Prosopagnosia: 2. Functional Neuroimaging Findings. J. Cogn. Neurosci. 2005, 17, 1150–1167. [Google Scholar] [CrossRef]
- Thomas, C.; Avidan, G.; Humphreys, K.; Jung, K.; Gao, F.; Behrmann, M. Reduced Structural Connectivity in Ventral Visual Cortex in Congenital Prosopagnosia. Nat. Neurosci. 2009, 12, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhen, Z.; Liu, X.; Song, Y.; Liu, J. The Neural Network for Face Recognition: Insights from an fMRI Study on Developmental Prosopagnosia. Neuroimage 2018, 169, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Avidan, G.; Tanzer, M.; Hadj-Bouziane, F.; Liu, N.; Ungerleider, L.G.; Behrmann, M. Selective Dissociation between Core and Extended Regions of the Face Processing Network in Congenital Prosopagnosia. Cereb. Cortex 2014, 24, 1565–1578. [Google Scholar] [CrossRef]
- DeGutis, J.M.; Bentin, S.; Robertson, L.C.; D’Esposito, M. Functional Plasticity in Ventral Temporal Cortex Following Cognitive Rehabilitation of a Congenital Prosopagnosic. J. Cogn. Neurosci. 2007, 19, 1790–1802. [Google Scholar] [CrossRef]
- Dinkelacker, V.; Grüter, M.; Klaver, P.; Grüter, T.; Specht, K.; Weis, S.; Kennerknecht, I.; Elger, C.E.; Fernandez, G. Congenital Prosopagnosia: Multistage Anatomical and Functional Deficits in Face Processing Circuitry. J. Neurol. 2011, 258, 770–782. [Google Scholar] [CrossRef]
- Furl, N.; Garrido, L.; Dolan, R.J.; Driver, J.; Duchaine, B. Fusiform Gyrus Face Selectivity Relates to Individual Differences in Facial Recognition Ability. J. Cogn. Neurosci. 2011, 23, 1723–1740. [Google Scholar] [CrossRef]
- Gerlach, C.; Klargaard, S.K.; Alnæs, D.; Kolskår, K.K.; Karstoft, J.; Westlye, L.T.; Starrfelt, R. Left Hemisphere Abnormalities in Developmental Prosopagnosia When Looking at Faces but Not Words. Brain Commun. 2019, 1, fcz034. [Google Scholar] [CrossRef]
- Gilaie-Dotan, S.; Perry, A.; Bonneh, Y.; Malach, R.; Bentin, S. Seeing with Profoundly Deactivated Mid-Level Visual Areas: Non-Hierarchical Functioning in the Human Visual Cortex. Cereb. Cortex 2009, 19, 1687–1703. [Google Scholar] [CrossRef]
- Hadjikhani, N.; de Gelder, B. Neural Basis of Prosopagnosia: An fMRI Study. Hum. Brain Mapp. 2002, 16, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jiahui, G.; Yang, H.; Duchaine, B. Attentional Modulation Differentially Affects Ventral and Dorsal Face Areas in Both Normal Participants and Developmental Prosopagnosics. Cogn. Neuropsychol. 2020, 37, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Garrido, L.; Driver, J.; Dolan, R.J.; Duchaine, B.C.; Furl, N. Effective Connectivity from Early Visual Cortex to Posterior Occipitotemporal Face Areas Supports Face Selectivity and Predicts Developmental Prosopagnosia. J. Neurosci. 2016, 36, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Minnebusch, D.A.; Daum, I. Neuropsychological Mechanisms of Visual Face and Body Perception. Neurosci. Biobehav. Rev. 2009, 33, 1133–1144. [Google Scholar] [CrossRef]
- Németh, K.; Zimmer, M.; Nagy, K.; Bankó, É.M.; Vidnyanszky, Z.; Vakli, P.; Kovács, G. Altered Bold Response Within the Core Face-Processing Network in Congenital Prosopagnosia. Ideggyógyászati Szle. 2015, 68, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, R.; Zhao, Y.; Zhen, Z.; Song, Y.; Liu, J. Multi-Item Discriminability Pattern to Faces in Developmental Prosopagnosia Reveals Distinct Mechanisms of Face Processing. Cereb. Cortex 2020, 30, 2986–2996. [Google Scholar] [CrossRef]
- Rosenthal, G.; Tanzer, M.; Simony, E.; Hasson, U.; Behrmann, M.; Avidan, G. Altered Topology of Neural Circuits in Congenital Prosopagnosia. Elife 2017, 6, e25069. [Google Scholar] [CrossRef]
- Rivolta, D.; Woolgar, A.; Palermo, R.; Butko, M.; Schmalzl, L.; Williams, M.A. Multi-Voxel Pattern Analysis (MVPA) Reveals Abnormal fMRI Activity in Both the “Core” and “Extended” Face Network in Congenital Prosopagnosia. Front. Hum. Neurosci. 2014, 8, 925. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Xu, Y. Neural Decoding Reveals Impaired Face Configural Processing in the Right Fusiform Face Area of Individuals with Developmental Prosopagnosia. J. Neurosci. 2015, 35, 1539–1548. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, F.; Song, Y.; Liu, J. Multiple-Stage Impairments of Unfamiliar Face Learning in Developmental Prosopagnosia: Evidence from fMRI Repetition Suppression and Multi-Voxel Pattern Stability. Neuropsychologia 2022, 176, 108370. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Song, Y.; Liu, J. Separate and Shared Neural Basis of Face Memory and Face Perception in Developmental Prosopagnosia. Front. Behav. Neurosci. 2021, 15, 668174. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.; Pestilli, F.; Witthoft, N.; Golarai, G.; Liberman, A.; Poltoratski, S.; Yoon, J.; Grill-Spector, K. Functionally Defined White Matter Reveals Segregated Pathways in Human Ventral Temporal Cortex Associated with Category-Specific Processing. Neuron 2015, 85, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Grossi, D.; Soricelli, A.; Ponari, M.; Salvatore, E.; Quarantelli, M.; Prinster, A.; Trojano, L. Structural Connectivity in a Single Case of Progressive Prosopagnosia: The Role of the Right Inferior Longitudinal Fasciculus. Cortex 2014, 56, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Garrido, L.; Nagy, Z.; Mohammadi, S.; Steel, A.; Driver, J.; Dolan, R.J.; Duchaine, B.; Furl, N. Local but Not Long-Range Microstructural Differences of the Ventral Temporal Cortex in Developmental Prosopagnosia. Neuropsychologia 2015, 78, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Avidan, G.; Behrmann, M. Functional MRI Reveals Compromised Neural Integrity of the Face Processing Network in Congenital Prosopagnosia. Curr. Biol. 2009, 19, 1146–1150. [Google Scholar] [CrossRef]
- Haeger, A.; Pouzat, C.; Luecken, V.; N’diaye, K.; Elger, C.; Kennerknecht, I.; Axmacher, N.; Dinkelacker, V. Face Processing in Developmental Prosopagnosia: Altered Neural Representations in the Fusiform Face Area. Front. Behav. Neurosci. 2021, 15, 744466. [Google Scholar] [CrossRef]
- Van den Stock, J.; van de Riet, W.A.; Righart, R.; de Gelder, B. Neural Correlates of Perceiving Emotional Faces and Bodies in Developmental Prosopagnosia: An Event-Related fMRI-Study. PLoS ONE 2008, 3, e3195. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Liu, X.; Song, Y.; Wang, R.; Yang, Z.; Liu, J. Altered Spontaneous Neural Activity in the Occipital Face Area Reflects Behavioral Deficits in Developmental Prosopagnosia. Neuropsychologia 2016, 89, 344–355. [Google Scholar] [CrossRef]
- Behrmann, M.; Avidan, G.; Gao, F.; Black, S. Structural Imaging Reveals Anatomical Alterations in Inferotemporal Cortex in Congenital Prosopagnosia. Cereb. Cortex 2007, 17, 2354–2363. [Google Scholar] [CrossRef]
- Bentin, S.; Deouell, L.Y.; Soroker, N. Selective Visual Streaming in Face Recognition: Evidence from Developmental Prosopagnosia. Neuroreport 1999, 10, 823–827. [Google Scholar] [CrossRef]
- Garrido, L.; Furl, N.; Draganski, B.; Weiskopf, N.; Stevens, J.; Tan, G.C.-Y.; Driver, J.; Dolan, R.J.; Duchaine, B. Voxel-Based Morphometry Reveals Reduced Grey Matter Volume in the Temporal Cortex of Developmental Prosopagnosics. Brain 2009, 132, 3443–3455. [Google Scholar] [CrossRef] [PubMed]
- Van den Stock, J.; Vandenbulcke, M.; Zhu, Q.; Hadjikhani, N.; De Gelder, B. Developmental Prosopagnosia in a Patient with Hypoplasia of the Vermis Cerebelli. Neurology 2012, 78, 1700–1702. [Google Scholar] [CrossRef] [PubMed]
- Bentin, S.; DeGutis, J.M.; D’Esposito, M.; Robertson, L.C. Too Many Trees to See the Forest: Performance, Event-Related Potential, and Functional Magnetic Resonance Imaging Manifestations of Integrative Congenital Prosopagnosia. J. Cogn. Neurosci. 2007, 19, 132–146. [Google Scholar] [CrossRef]
- Burns, E.J.; Tree, J.J.; Weidemann, C.T. Recognition Memory in Developmental Prosopagnosia: Electrophysiological Evidence for Abnormal Routes to Face Recognition. Front. Hum. Neurosci. 2014, 8, 622. [Google Scholar] [CrossRef]
- Collins, E.; Dundas, E.; Gabay, Y.; Plaut, D.C.; Behrmann, M. Hemispheric Organization in Disorders of Development. Vis. Cogn. 2017, 25, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Kress, T.; Daum, I. Event-Related Potentials Reflect Impaired Face Recognition in Patients with Congenital Prosopagnosia. Neurosci. Lett. 2003, 352, 133–136. [Google Scholar] [CrossRef]
- Lueschow, A.; Weber, J.E.; Carbon, C.-C.; Deffke, I.; Sander, T.; Grüter, T.; Grüter, M.; Trahms, L.; Curio, G. The 170ms Response to Faces as Measured by MEG (M170) Is Consistently Altered in Congenital Prosopagnosia. PLoS ONE 2015, 10, e0137624. [Google Scholar] [CrossRef]
- Minnebusch, D.A.; Suchan, B.; Ramon, M.; Daum, I. Event-Related Potentials Reflect Heterogeneity of Developmental Prosopagnosia. Eur. J. Neurosci. 2007, 25, 2234–2247. [Google Scholar] [CrossRef]
- Németh, K.; Zimmer, M.; Schweinberger, S.R.; Vakli, P.; Kovács, G. The Background of Reduced Face Specificity of N170 in Congenital Prosopagnosia. PLoS ONE 2014, 9, e101393. [Google Scholar] [CrossRef]
- Towler, J.; Gosling, A.; Duchaine, B.; Eimer, M. The Face-Sensitive N170 Component in Developmental Prosopagnosia. Neuropsychologia 2012, 50, 3588–3599. [Google Scholar] [CrossRef] [PubMed]
- Righart, R.; de Gelder, B. Impaired Face and Body Perception in Developmental Prosopagnosia. Proc. Natl. Acad. Sci. USA 2007, 104, 17234–17238. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Towler, J.; Eimer, M. Face Identity Matching Is Selectively Impaired in Developmental Prosopagnosia. Cortex 2017, 89, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Towler, J.; Fisher, K.; Eimer, M. Holistic Face Perception Is Impaired in Developmental Prosopagnosia. Cortex 2018, 108, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Eimer, M.; Gosling, A.; Duchaine, B. Electrophysiological Markers of Covert Face Recognition in Developmental Prosopagnosia. Brain 2012, 135, 542–554. [Google Scholar] [CrossRef]
- Parketny, J.; Towler, J.; Eimer, M. The Activation of Visual Face Memory and Explicit Face Recognition Are Delayed in Developmental Prosopagnosia. Neuropsychologia 2015, 75, 538–547. [Google Scholar] [CrossRef]
- Fisher, K.; Towler, J.; Rossion, B.; Eimer, M. Neural Responses in a Fast Periodic Visual Stimulation Paradigm Reveal Domain-General Visual Discrimination Deficits in Developmental Prosopagnosia. Cortex 2020, 133, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Olivares, E.I.; Urraca, A.S.; Lage-Castellanos, A.; Iglesias, J. Different and Common Brain Signals of Altered Neurocognitive Mechanisms for Unfamiliar Face Processing in Acquired and Developmental Prosopagnosia. Cortex 2021, 134, 92–113. [Google Scholar] [CrossRef]
- Dobel, C.; Junghöfer, M.; Gruber, T. The Role of Gamma-Band Activity in the Representation of Faces: Reduced Activity in the Fusiform Face Area in Congenital Prosopagnosia. PLoS ONE 2011, 6, e19550. [Google Scholar] [CrossRef]
- Rivolta, D.; Palermo, R.; Schmalzl, L.; Williams, M.A. Investigating the Features of the M170 in Congenital Prosopagnosia. Front. Hum. Neurosci. 2012, 6, 45. [Google Scholar] [CrossRef]
- Dobel, C.; Putsche, C.; Zwitserlood, P.; Junghöfer, M. Early Left-Hemispheric Dysfunction of Face Processing in Congenital Prosopagnosia: An MEG Study. PLoS ONE 2008, 3, e2326. [Google Scholar] [CrossRef]
- Harris, A.M.; Duchaine, B.C.; Nakayama, K. Normal and Abnormal Face Selectivity of the M170 Response in Developmental Prosopagnosics. Neuropsychologia 2005, 43, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, M.; Avidan, G. Congenital Prosopagnosia: Face-Blind from Birth. Trends Cogn. Sci. 2005, 9, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.J.; Iaria, G.; Barton, J.J. Defining the Face Processing Network: Optimization of the Functional Localizer in fMRI. Hum. Brain Mapp. 2009, 30, 1637–1651. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.J. Structure and Function in Acquired Prosopagnosia: Lessons from a Series of 10 Patients with Brain Damage. J. Neuropsychol. 2008, 2, 197–225. [Google Scholar] [CrossRef] [PubMed]
- Sorger, B.; Goebel, R.; Schiltz, C.; Rossion, B. Understanding the Functional Neuroanatomy of Acquired Prosopagnosia. Neuroimage 2007, 35, 836–852. [Google Scholar] [CrossRef]
- Martin, A.; Chao, L.L. Semantic Memory and the Brain: Structure and Processes. Curr. Opin. Neurobiol. 2001, 11, 194–201. [Google Scholar] [CrossRef]
- Yovel, G.; Tambini, A.; Brandman, T. The Asymmetry of the Fusiform Face Area Is a Stable Individual Characteristic That Underlies the Left-Visual-Field Superiority for Faces. Neuropsychologia 2008, 46, 3061–3068. [Google Scholar] [CrossRef]
- Peelen, M.V.; Downing, P.E. Within-Subject Reproducibility of Category-Specific Visual Activation with Functional MRI. Hum. Brain Mapp. 2005, 25, 402–408. [Google Scholar] [CrossRef]
- Weiner, K.S.; Grill-Spector, K. Sparsely-Distributed Organization of Face and Limb Activations in Human Ventral Temporal Cortex. Neuroimage 2010, 52, 1559–1573. [Google Scholar] [CrossRef]
- Weiner, K.S.; Golarai, G.; Caspers, J.; Chuapoco, M.R.; Mohlberg, H.; Zilles, K.; Amunts, K.; Grill-Spector, K. The Mid-Fusiform Sulcus: A Landmark Identifying Both Cytoarchitectonic and Functional Divisions of Human Ventral Temporal Cortex. Neuroimage 2014, 84, 453–465. [Google Scholar] [CrossRef]
- Rossion, B.; Jacques, C. Does Physical Interstimulus Variance Account for Early Electrophysiological Face Sensitive Responses in the Human Brain? Ten Lessons on the N170. Neuroimage 2008, 39, 1959–1979. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, N.; Bentin, S. Structural Encoding of Human and Schematic Faces: Holistic and Part-Based Processes. J. Cogn. Neurosci. 2001, 13, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, R.; Cooper, P.A.; Mondloch, C.J.; Lewis, T.L.; Sagiv, N.; de Gelder, B.; Maurer, D. What Aspects of Face Processing Are Impaired in Developmental Prosopagnosia? Brain Cogn. 2006, 61, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Delvenne, J.-F.; Debatisse, D.; Goffaux, V.; Bruyer, R.; Crommelinck, M.; Guérit, J.-M. Spatio-Temporal Localization of the Face Inversion Effect: An Event-Related Potentials Study. Biol. Psychol. 1999, 50, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Gauthier, I.; Tarr, M.J.; Despland, P.; Bruyer, R.; Linotte, S.; Crommelinck, M. The N170 Occipito-Temporal Component Is Delayed and Enhanced to Inverted Faces but Not to Inverted Objects: An Electrophysiological Account of Face-Specific Processes in the Human Brain. Neuroreport 2000, 11, 69–72. [Google Scholar] [CrossRef]
- Bonemei, R.; Costantino, A.I.; Battistel, I.; Rivolta, D. The Perception of (Naked Only) Bodies and Faceless Heads Relies on Holistic Processing: Evidence from the Inversion Effect. Br. J. Psychol. 2018, 109, 232–243. [Google Scholar] [CrossRef]
- Aviezer, H.; Trope, Y.; Todorov, A. Holistic Person Processing: Faces with Bodies Tell the Whole Story. J. Personal. Soc. Psychol. 2012, 103, 20. [Google Scholar] [CrossRef]
- Rivolta, D.; Palermo, R.; Schmalzl, L.; Coltheart, M. Covert Face Recognition in Congenital Prosopagnosia: A Group Study. Cortex 2012, 48, 344–352. [Google Scholar] [CrossRef]
- Breen, N.; Caine, D.; Coltheart, M.; Hendy, J.; Roberts, C. Towards an Understanding of Delusions of Misidentification: Four Case Studies. Mind Lang. 2000, 15, 74–110. [Google Scholar] [CrossRef]
- Bruce, V.; Young, A. Understanding Face Recognition. Br. J. Psychol. 1986, 77, 305–327. [Google Scholar] [CrossRef]
- Rivolta, D.; Palermo, R.; Schmalzl, L.; Williams, M.A. An Early Category-Specific Neural Response for the Perception of Both Places and Faces. Cogn. Neurosci. 2012, 3, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Higuchi, M.; Marantz, A.; Kanwisher, N. The Selectivity of the Occipitotemporal M170 for Faces. Neuroreport 2000, 11, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Deffke, I.; Sander, T.; Heidenreich, J.; Sommer, W.; Curio, G.; Trahms, L.; Lueschow, A. MEG/EEG Sources of the 170-Ms Response to Faces Are Co-Localized in the Fusiform Gyrus. Neuroimage 2007, 35, 1495–1501. [Google Scholar] [CrossRef]
- Gruber, T.; Trujillo-Barreto, N.J.; Giabbiconi, C.-M.; Valdés-Sosa, P.A.; Müller, M.M. Brain Electrical Tomography (BET) Analysis of Induced Gamma Band Responses during a Simple Object Recognition Task. NeuroImage 2006, 29, 888–900. [Google Scholar] [CrossRef]
- Gonzalez-Perez, M.; Wakui, E.; Thoma, V.; Nitsche, M.A.; Rivolta, D. Transcranial Alternating Current Stimulation (tACS) at 40 Hz Enhances Face and Object Perception. Neuropsychologia 2019, 135, 107237. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Chiarantoni, G.; Bossi, F.; Conti, A.; D’Elia, V.; Tagliente, S.; Nitsche, M.A.; Rivolta, D. Face Pareidolia Is Enhanced by 40 Hz Transcranial Alternating Current Stimulation (tACS) of the Face Perception Network. Sci. Rep. 2023, 13, 2035. [Google Scholar] [CrossRef]
- Zion-Golumbic, E.; Golan, T.; Anaki, D.; Bentin, S. Human Face Preference in Gamma-Frequency EEG Activity. Neuroimage 2008, 39, 1980–1987. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory Gamma Activity in Humans and Its Role in Object Representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef]
- Schmalzl, L.; Palermo, R.; Coltheart, M. Cognitive Heterogeneity in Genetically Based Prosopagnosia: A Family Study. J. Neuropsychol. 2008, 2, 99–117. [Google Scholar] [CrossRef]
- Barton, J.J.S.; Corrow, S.L. The Problem of Being Bad at Faces. Neuropsychologia 2016, 89, 119–124. [Google Scholar] [CrossRef]
- Duchaine, B.; Germine, L.; Nakayama, K. Family Resemblance: Ten Family Members with Prosopagnosia and within-Class Object Agnosia. Cogn. Neuropsychol. 2007, 24, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Geskin, J.; Behrmann, M. Congenital Prosopagnosia without Object Agnosia? A Literature Review. Cogn. Neuropsychol. 2018, 35, 4–54. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.-D.; Rees, G. Decoding Mental States from Brain Activity in Humans. Nat. Rev. Neurosci. 2006, 7, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.A.; Polyn, S.M.; Detre, G.J.; Haxby, J.V. Beyond Mind-Reading: Multi-Voxel Pattern Analysis of fMRI Data. Trends Cogn. Sci. 2006, 10, 424–430. [Google Scholar] [CrossRef]
- Mur, M.; Bandettini, P.A.; Kriegeskorte, N. Revealing Representational Content with Pattern-Information fMRI—An Introductory Guide. Soc. Cogn. Affect. Neurosci. 2009, 4, 101–109. [Google Scholar] [CrossRef]
- Ojemann, J.G.; Akbudak, E.; Snyder, A.Z.; McKinstry, R.C.; Raichle, M.E.; Conturo, T.E. Anatomic Localization and Quantitative Analysis of Gradient Refocused Echo-Planar fMRI Susceptibility Artifacts. NeuroImage 1997, 6, 156–167. [Google Scholar] [CrossRef]
- Kennerknecht, I.; Ho, N.Y.; Wong, V.C.N. Prevalence of Hereditary Prosopagnosia (HPA) in Hong Kong Chinese Population. Am. J. Med. Genet. A 2008, 146A, 2863–2870. [Google Scholar] [CrossRef]
- Kennerknecht, I.; Grueter, T.; Welling, B.; Wentzek, S.; Horst, J.; Edwards, S.; Grueter, M. First Report of Prevalence of Non-Syndromic Hereditary Prosopagnosia (HPA). Am. J. Med. Genet. A 2006, 140, 1617–1622. [Google Scholar] [CrossRef]
- Duchaine, B.C.; Yovel, G.; Butterworth, E.J.; Nakayama, K. Prosopagnosia as an Impairment to Face-Specific Mechanisms: Elimination of the Alternative Hypotheses in a Developmental Case. Cogn. Neuropsychol. 2006, 23, 714–747. [Google Scholar] [CrossRef]
- Ramus, F. Neurobiology of Dyslexia: A Reinterpretation of the Data. Trends Neurosci. 2004, 27, 720–726. [Google Scholar] [CrossRef]
- Rossion, B.; Caldara, R.; Seghier, M.; Schuller, A.-M.; Lazeyras, F.; Mayer, E. A Network of Occipito-Temporal Face-Sensitive Areas besides the Right Middle Fusiform Gyrus Is Necessary for Normal Face Processing. Brain 2003, 126, 2381–2395. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Lochy, A. Is Human Face Recognition Lateralized to the Right Hemisphere Due to Neural Competition with Left-Lateralized Visual Word Recognition? A Critical Review. Brain Struct. Funct. 2022, 227, 599–629. [Google Scholar] [CrossRef]
- Thome, I.; García Alanis, J.C.; Volk, J.; Vogelbacher, C.; Steinsträter, O.; Jansen, A. Let’s Face It: The Lateralization of the Face Perception Network as Measured with fMRI Is Not Clearly Right Dominant. NeuroImage 2022, 263, 119587. [Google Scholar] [CrossRef] [PubMed]
- Brancucci, A.; Ferracci, S.; D’Anselmo, A.; Manippa, V. Hemispheric Functional Asymmetries and Sex Effects in Visual Bistable Perception. Conscious. Cogn. 2023, 113, 103551. [Google Scholar] [CrossRef] [PubMed]
- Proverbio, A.M.; Brignone, V.; Matarazzo, S.; Del Zotto, M.; Zani, A. Gender Differences in Hemispheric Asymmetry for Face Processing. BMC Neurosci. 2006, 7, 44. [Google Scholar] [CrossRef]
- Russell, R.; Duchaine, B.; Nakayama, K. Super-Recognizers: People with Extraordinary Face Recognition Ability. Psychon. Bull. Rev. 2009, 16, 252–257. [Google Scholar] [CrossRef]
- Burns, E.J.; Arnold, T.; Bukach, C.M. P-Curving the Fusiform Face Area: Meta-Analyses Support the Expertise Hypothesis. Neurosci. Biobehav. Rev. 2019, 104, 209–221. [Google Scholar] [CrossRef]
- McGugin, R.W.; Gauthier, I. Perceptual Expertise with Objects Predicts Another Hallmark of Face Perception. J. Vis. 2009, 10, 15. [Google Scholar] [CrossRef]
- Bobak, A.K.; Bennetts, R.J.; Parris, B.A.; Jansari, A.; Bate, S. An In-Depth Cognitive Examination of Individuals with Superior Face Recognition Skills. Cortex 2016, 82, 48–62. [Google Scholar] [CrossRef]
- Bobak, A.K.; Parris, B.A.; Gregory, N.J.; Bennetts, R.J.; Bate, S. Eye-Movement Strategies in Developmental Prosopagnosia and “Super” Face Recognition. Q. J. Exp. Psychol. 2017, 70, 201–217. [Google Scholar] [CrossRef]
- DeGutis, J.; Cohan, S.; Mercado, R.J.; Wilmer, J.; Nakayama, K. Holistic Processing of the Mouth but Not the Eyes in Developmental Prosopagnosia. Cogn. Neuropsychol. 2012, 29, 419–446. [Google Scholar] [CrossRef] [PubMed]
- DeGutis, J.; Chatterjee, G.; Mercado, R.J.; Nakayama, K. Face Gender Recognition in Developmental Prosopagnosia: Evidence for Holistic Processing and Use of Configural Information. Vis. Cogn. 2012, 20, 1242–1253. [Google Scholar] [CrossRef]
- Dalrymple, K.A.; Garrido, L.; Duchaine, B. Dissociation between Face Perception and Face Memory in Adults, but Not Children, with Developmental Prosopagnosia. Dev. Cogn. Neurosci. 2014, 10, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, P.I.; Wilkinson, D.T.; Ferguson, H.J.; Smith, L.J.; Bindemann, M.; Johnston, R.A.; Schmalzl, L. Perceptual and Memorial Contributions to Developmental Prosopagnosia. Q. J. Exp. Psychol. 2017, 70, 298–315. [Google Scholar] [CrossRef] [PubMed]
- McKone, E.; Hall, A.; Pidcock, M.; Palermo, R.; Wilkinson, R.B.; Rivolta, D.; Yovel, G.; Davis, J.M.; O’Connor, K.B. Face Ethnicity and Measurement Reliability Affect Face Recognition Performance in Developmental Prosopagnosia: Evidence from the Cambridge Face Memory Test–Australian. Cogn. Neuropsychol. 2011, 28, 109–146. [Google Scholar] [CrossRef]
- DeGutis, J.M.; Chiu, C.; Grosso, M.E.; Cohan, S. Face Processing Improvements in Prosopagnosia: Successes and Failures over the Last 50 Years. Front. Hum. Neurosci. 2014, 8, 561. [Google Scholar] [CrossRef]
- DeGutis, J.; Cohan, S.; Nakayama, K. Holistic Face Training Enhances Face Processing in Developmental Prosopagnosia. Brain 2014, 137, 1781–1798. [Google Scholar] [CrossRef]
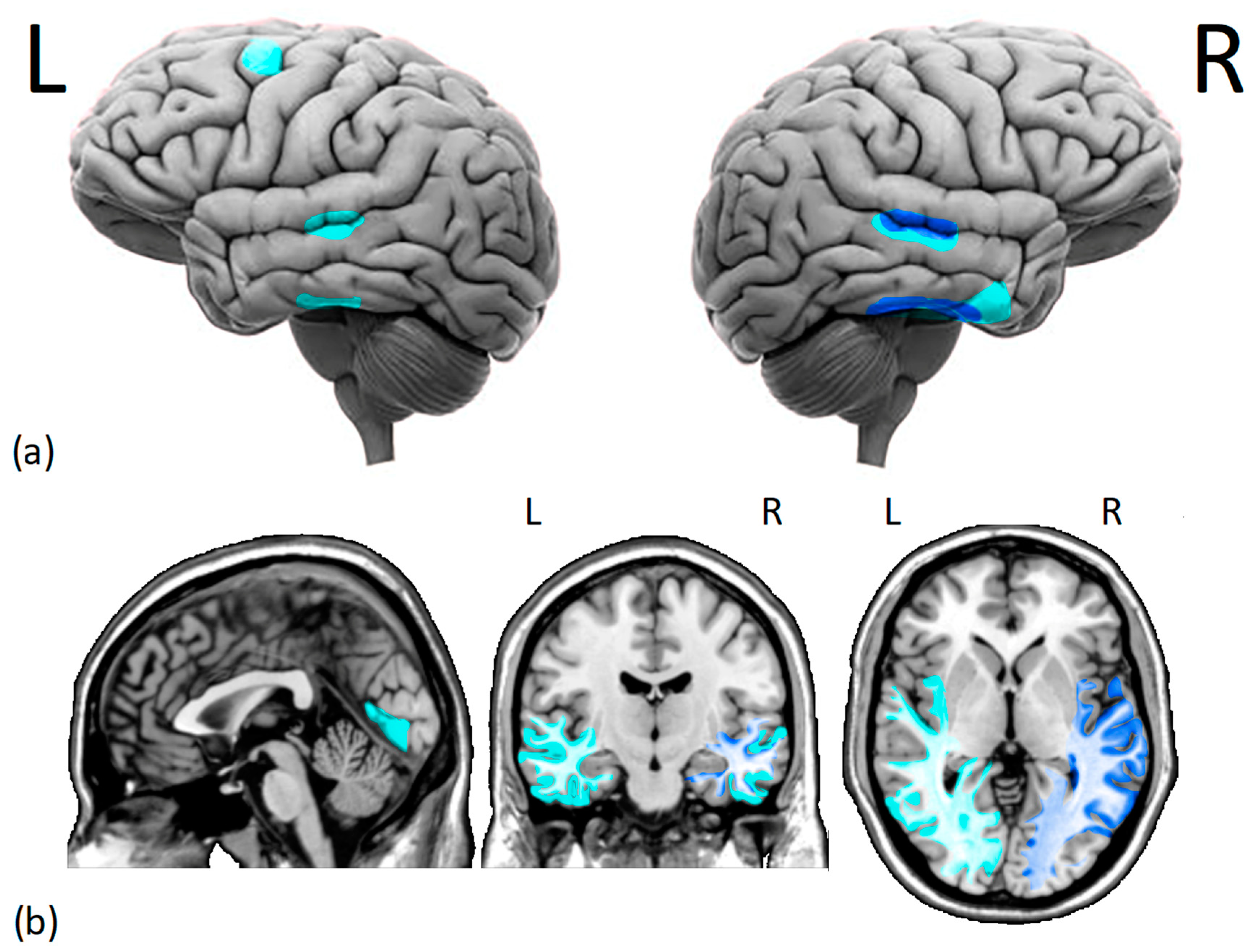
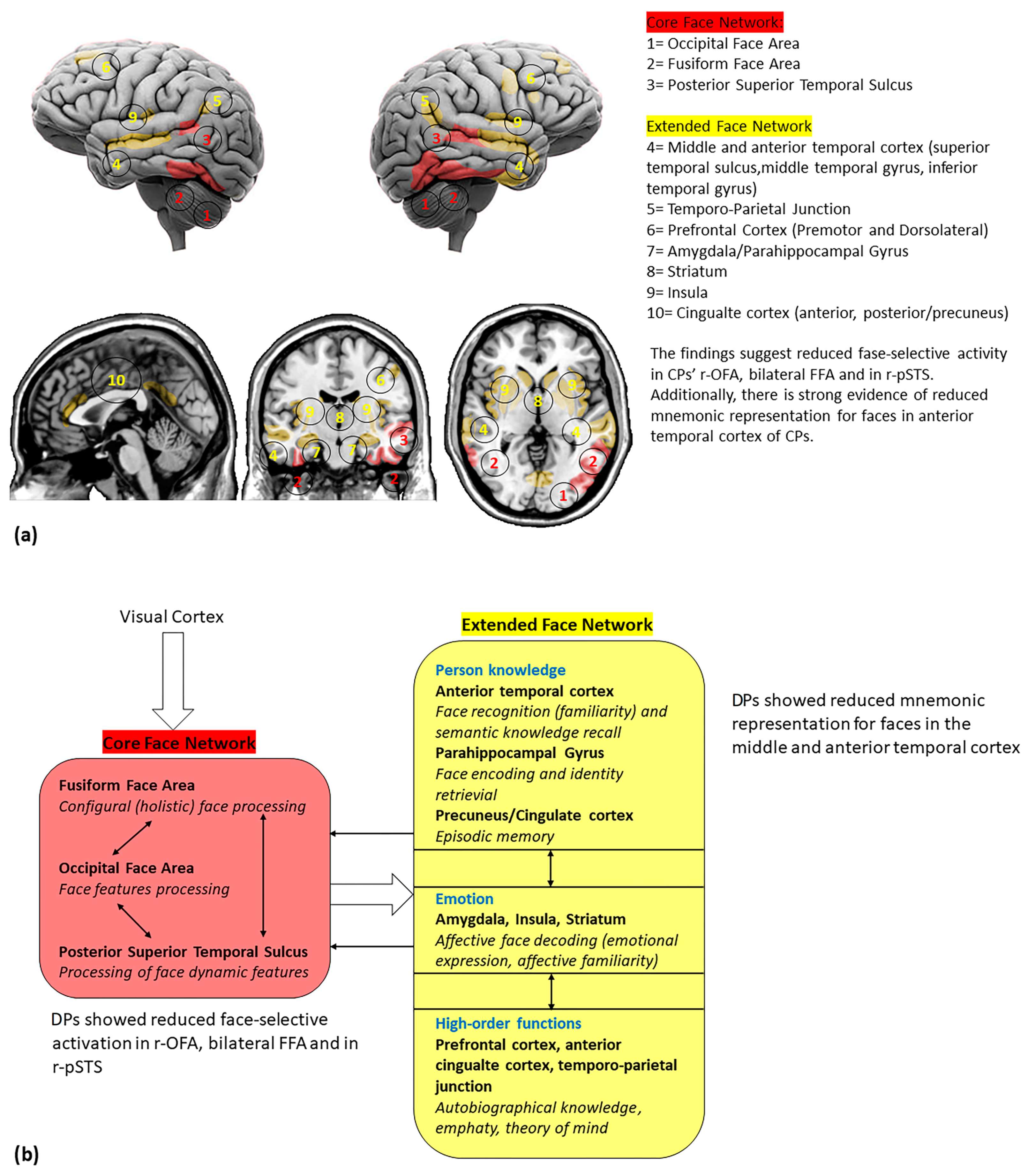
| DPs Sample Size | sMRI N | fMRI N | DTI N | fc-fMRI N | EEG N | MEG N | Tot N | % |
|---|---|---|---|---|---|---|---|---|
| Single case | 4 | 4 | 1 | 0 | 5 | 0 | 14 | 22.22 |
| ≤5 | 0 | 4 | 0 | 0 | 4 | 1 | 9 | 14.29 |
| 6 ≥ 10 | 1 | 5 | 1 | 1 | 4 | 3 | 15 | 23.81 |
| 11 ≥ 20 | 2 | 5 | 2 | 1 | 6 | 1 | 17 | 26.98 |
| ≥21 | 1 | 4 | 0 | 3 | 0 | 0 | 8 | 12.70 |
| Tot | 8 | 22 | 4 | 5 | 19 | 5 | 63 | 100.00 |
| Protocol/ Outcomes | Reference | Participants | Task Administered during the Protocol | Main Findings | Diagnosis and Behavioral Data |
|---|---|---|---|---|---|
| Block fMRI | Avidan et al., 2005 [51] | 4 DPs, 10 HCs | (1) One-back task (face vs. object); (2) motion pictures passive viewing (face vs. object); (3) adaptation (face vs. object); (4) Rubin’s face–vase | DPs showed normal fMRI activation in the FFA and the rest of VOTC during both face and non-face stimuli viewing. DPs also showed normal adaptation levels like HCs and exhibited evidence of global face representation in the FFA. | DPs scored significantly lower than HCs in recognizing familiar faces and in the same/different discrimination judgments on unfamiliar faces. DPs were also impaired, although to a lesser extent and with greater variability, on tasks involving nonface stimuli. All DPs performed within the normal range on various low-level visual processing tasks. |
| Block fMRI | Avidan et al., 2014 [56] | 7 DPs, 7 HCs | Passive viewing (emotional faces, neutral faces, famous faces, unfamiliar faces, or buildings) | DPs showed normal activation patterns and functional connectivity in the core face networks and the amygdala. | DPs reported substantial life-long difficulties with face processing. DPs scored below (2 SD) the normal range on at least 2 out of 4 different diagnostic tests (FFT, CFMT, CFPT, discrimination of novel upright and inverted faces). |
| Block fMRI | DeGutis et al., 2007 [57] | 1 DPs, 24 HCs | One-back task (faces, scenes); face classification training | The DP case showed face-selective activation in bilateral FFA and r- OFA. After the training, the functional connectivity between FFA and OFA increased. | The DP case was severely impaired in face recognition and classification, whereas she performed within the normal range on one-back face task and in object and word recognition. Following training, DP case’s performance at the face classification task improved. |
| Block fMRI | Dinkelacker et al., 2011 [58] | 24 DPs, 25 HCs | Discrimination task (neutral, positive, negative emotional faces, building, and scrambled faces) | DPs showed decreased signal during face processing in the FG, LOC, and right DLPFC. Processing of buildings was accompanied by decreased brain activity in the MTG, ventral, and precentral DLPFC. | DPs were impaired in long-term memory for faces and, to a lesser extent, other complex visual stimuli such as buildings. However, their memory for faces with negative valence and the ability to categorize emotional expressions were preserved. |
| Block fMRI | Furl et al., 2011 [59] | 15 DPs, 15 HCs | Passive viewing (cars, faces) | DPs showed reduced face-selective responses in the bilateral FFA and smaller face-selective clusters in the r-FFA. A robust relationship between face selectivity and face identification ability was found in FG across all the samples. | DPs reported substantial life-long difficulties with face processing. DPs scored below (2 SD) the normal range on the FFT and the CFMT. DPs did not differ from HCs in IQ scores and low-level perceptual abilities. |
| Block fMRI | Gerlach et al., 2019 [60] | 15 DPs, 34 HCs | Target detection task (faces, houses, tools, and words) | DPs showed reduced activation of core ventral face areas during perception of faces (more pronounced in the left hemisphere). No differences were found in activation to orthographic material and objects. | DPs reported substantial life-long difficulties with face processing. DPs scored below HCs in CFMT and a face identification task. |
| Block fMRI | Gilaie-Dotan et al., 2009 [61] | 1 DP, 9 HCs | (1) Category-selectivity mapping (faces, houses, objects, and patterns); (2) motion-selectivity mapping (static, motion); (3) completion experiment | The secondary visual cortex (V2-V4) was strongly deactivated in DPs compared with HCs, whereas activity in the primary visual cortex and the VOTC was robust, with selectivity for faces and objects impaired mainly in the left hemisphere. | Face processing was extremely impaired in the DP case; no other kind of low- and high-level vision deficits were observed. |
| Block fMRI | Hasson et al., 2003 [50] | 1 DP, 12 HCs | One-back task (faces, buildings, objects, geometric patterns); Exp. 2: Passive view (modified Rubin’s face–vase, lines drawing a face, and goblets) | DP’s face-related activation pattern in the VOTC was similar to that observed in HCs. | DP case was impaired on the FFT but was able to identify gender, age, and emotional state based on a person’s face. He had no difficulty in recognizing objects and exhibited normal performance in holistic and analytic visual, memory, and cognitive tests. |
| Block fMRI | Hadjikhai & de Gelder, 2002 [62] | 1 DP, 2 HCs | Passive viewing (faces, objects, houses, scenes) | DP showed no face-selective activation in the FFA and OFA. | N/A. |
| Block fMRI | Jiahui et al., 2020 [63] | 12 DPs, 16 HCs | One-back task | FFA and OFA responded strongly both in HCs and DPs when attention was directed to identity and expression, while pSTS and IFG responded the most when attention was directed to facial expression. | DPs reported problems with face recognition in daily life and scored below (1 SD) the normal range on at least 3 different diagnostic tests (CFMT, FFT, and an O/NFT). Expression recognition ability was preserved. |
| Block fMRI (dynamic causal modeling) | Lohse et al., 2016 [64] | 15 DPs, 15 HCs | Passive viewing with repetitions (emotional faces and cars) | DPs exhibit reduced strength of feed-forward connections carrying face information from the early visual cortex to FFA and pSTS. These network alterations contribute to the diminished face selectivity in the posterior occipitotemporal areas affected in DP. This response profile was comparable in DPs and HCs. | DPs reported substantial life-long difficulties with face processing. DPs scored below (2 SD) the normal range on the FFT and the CFMT. DPs did not differ from HCs in IQ scores and low-level perceptual abilities. |
| Block fMRI | Minnebusch & Daum, 2009 [65] | 4 DPs, 7 HCs | Passive viewing (famous, non-famous faces, caricatures and non-face objects) | HCs showed higher face-related activations in the r- compared to the l-FFA, with higher activation in the OFA and FFA for new faces vs. known faces. Contrary to HCs, DPs did not show bilateral face-related activations in the OFA and FFA. | DPs face recognition was heterogeneously impaired with normal basic visual processing and intact object recognition abilities. |
| Block fMRI | Németh et al., 2015 [66] | 3 DPs, 20 HCs | One-back task on faces and nonsense objects and verbal report of the total number of one-back repetitions at the end of each run (total runs = 6) | FFA and OFA activity, as well as LOC activity, was significantly reduced in DPs. Analysis of the hemodynamic response function revealed a lower peak response, but also a significantly faster and stronger decay of the VOTC response in DPs. | DPs showed impaired face recognition and perception capacities on the CFMT, whereas object recognition and IQ were in the normal range. |
| Block fMRI (multi-item discrimability pattern) | Tian et al., 2020 [67] | 64 DPs, 62 HCs | One-back task (faces, scenes, objects, and scrambled objects) | DPs’ r-FFA and OFA activation patterns for faces differed from the mean activation pattern of HCs. | DPs demonstrated face-specific impairments during 4 phases of a multiple-stage procedure, while low-level vision, multimodal person recognition, and general object recognition were intact. In the last stage, DPs scored below (1 SD) the normative range on a computer-based O/NFT and FFT. |
| Block fMRI (inter-subject functional correlation) | Rosenthal et al., 2017 [68] | 10 DPs, 10 HCs | Passive viewing (emotional faces, neutral faces, familiar faces, unfamiliar faces, and non-faces) | The aTL served as the major network hub for face processing in HCs but not in DPs, which showed hyper-connectivity in lateral occipital and the inferior temporal cortices. The extent of this hyper-connectivity was correlated with DPs’ face recognition deficit. | DPs scored below (2 SD) the normal range at least on 2 out of 4 different diagnostic tests (FFT, CFMT, CFPT, discrimination of novel upright and inverted faces). |
| Block fMRI (multi-voxel pattern analysis) | Rivolta et al., 2014 [69] | 7 DPs, 10 HCs | One-back task (faces, headless bodies, body parts, and objects) | Neural activity within the core and extended face regions in DPs showed reduced discriminability between faces and objects. Reduced differentiation between faces and objects in DP was also observed in the right parahippocampal cortex. | DPs scored below (2 SD) the normal range on at least 1 out of 3 diagnostic tests (FFT, CFMT and CFPT). DPs did not differ from HCs in IQ scores, low-level perceptual abilities, and object recognition. |
| Block fMRI (multi-voxel pattern analysis) | Zhang et al., 2015 [70] | 7 DPs, 21 HCs | Passive viewing (intact face, face features, scrambled face, and non-face stimuli) | Right FFA’s responded preferably to faces in DPs and HCs, but no distinct neural response patterns were observed in DPs for the intact and the scrambled face configurations. | DPs scored below (2 SD) the normal range on a computer-based O/NFDT and FFT. |
| Block fMRI (multi-voxel pattern analysis) | Zhao et al., 2022 [71] | 64 DPs, 62 HCs | Passive viewing with repetitions (faces, objects, scenes, or scrambled objects) | FFA in DPs showed attenuated repetition suppression for faces, suggesting an inefficient perceptual analysis for learned faces. At the mnemonic level, DPs showed decreased stability for repeated faces in MTG, suggesting an unstable mnemonic representation for learned faces, which was associated with impaired face recognition performance in DP. | DPs demonstrated face-specific impairments during 4 phases of a multiple-stage procedure, while low-level vision, multimodal person recognition, and general object recognition were intact. In the last stage, DPs scored below (1 SD) the normative range on a computer-based O/NFT and FFT. |
| Block fMRI (voxel-wise brain–behavior correlation analyses) | Liu et al., 2021 [72] | 64 DPs, 61 HCs | Passive viewing (faces, objects, scenes, and scrambled objects) | DPs’ face memory performance was linked to bilateral FFA activity, while right pSTS activity was associated with face perception. Deficits in both tasks shared neural substrates in r-precuneus and r-orbitofrontal cortex. | DPs demonstrated face-specific impairments during 4 phases of a multiple-stage procedure, while low-level vision, multimodal person recognition, and general object recognition were intact. In the last stage, DPs scored below (1 SD) the normative range on a computer-based O/NFT and FFT. |
| Diffusor Tensor Imaging | Gomez et al., 2015 [73] | 18 DPs, 18 HCs | None | DPs expressed an atypical tract structure-behavior relationship near face-selective regions. | DPs scored below (2 SD) the normal range on the CFMT. |
| Diffusor Tensor Imaging | Grossi et al., 2014 [74] | 1 DP, 7 HCs | None | The right inferior longitudinal fasciculus was markedly reduced in DPs. | DP case scored borderline on the BFRT and was able to refer the faces’ gender but was not able to distinguish famous from unclear faces and could neither name nor identify celebrities from their photographs in the FFT. DP case showed mild visual agnosia for living and man-made objects. The general neuropsychological assessment did not reveal impairments. |
| Diffusor Tensor Imaging | Song et al., 2015 [75] | 16 DPs, 16 HCs | None | ILF and IFOF white matter were comparable between DPs and HCs. DPs had lower fractional anisotropy in white matter local to the r-FFA. | DP case scored below (3 SD) the normal range on FFT. |
| Diffusor Tensor Imaging | Thomas et al., 2009 [52] | 6 DPs, 12 HCs | None | DPs showed a marked reduction in the structural integrity of the inferior longitudinal fasciculus and the inferior fronto-occipito fasciculus bilaterally compared with HCs. In addition, DPs showed a reduction in fractional anisotropy in the bilateral FG, right anterior temporal stem, and left external capsule white matter. | DPs scored significantly lower than HCs in recognizing familiar faces and in making same/different discrimination judgments on unfamiliar faces. DPs were also impaired, although to a lesser extent and with greater variability, on tasks involving non-face stimuli. All DPs performed within the normal range on various low-level visual processing tasks. |
| Event-related fMRI | Avidan & Behrmann, 2009 [76] | 6 DPs, 12 HCs | Familiarity identity judgment (famous vs. unknown X same vs. different) | The fMRI signal was greater in the HCs than in DPs, but normal face identity adaptation effects were observed in DPs. HCs, but not DPs, presented selective activation for familiar vs. unknown faces in the precuneus/posterior cingulate cortex and the anterior paracingulate cortex. | DPs scored significantly lower than HCs in recognizing familiar faces and making same/different discrimination judgments of unfamiliar faces. DPs were also impaired, although to a lesser extent and with greater variability, on tasks involving nonface stimuli. All DPs performed within the normal range on various low-level visual processing tasks. |
| Event-related fMRI | Haeger et al., 2021 [77] | 13 DPs, 12 HCs | Modified Sternberg paradigm (low, medium and high load) | DPs failed to generate robust and maintained neural representations in the FFA during face encoding and maintenance. | The diagnosis was based on a complex pattern of features, representing both clinical complaints of long-term memory deficits and compensatory strategies, CFMT score, and family history. The CFMT revealed a significant difference in accuracy between DPs and HCs. |
| Event-related fMRI | Van den Stock et al., 2008 [78] | 3 DPs, 4 HCs | Oddball detection task with pictures of fearful and happy expressions (body and faces), emotionally neutral body expressions, and houses | Neutral, but not emotional, faces triggered lower right FFA activation in the DPs compared with HCs. DPs showed stronger activation for bodies in the inferior occipital gyrus and for neutral faces in the extrastriate body area, indicating that body- and face-sensitive processes are less categorically segregated in DPs. | DPs scored below the normal range on the BFRT and/or the WFMT with a preserved visual recognition for non-face stimuli. |
| Functional connectivity fMRI (fractional amplitude of spontaneous low-frequency fluctuations and functional regional homogeneity) | Zhao et al., 2016 [79] | 64 DPs, 62 HCs | Resting state | Different aspects of abnormal spontaneous neural activity within r-OFA underlie DP face-processing deficit. | DPs demonstrated face-specific impairments during 4 phases of a multiple-stage procedure, while low-level vision, multimodal person recognition and general object recognition were intact. In the last stage, DPs scored below (1 SD) the normative range on a computer-based O/NFT and FFT. |
| Functional connectivity fMRI | Avidan et al., 2014 [56] | 7 DPs, 7 DPs | Visual stimulation/resting state | A typical connectivity pattern was observed in the core face network of the DPs, while diminished connectivity in the pSTS, r-aTC, and augmented connectivity of the r-amygdala were found in DPs compared with HCs. | DPs reported substantial life-long difficulties with face processing. DPs scored below (2 SD) the normal range at least at 2 out of 4 different experiments: the FFT, the CFMT, the CFPT, and a task measuring discrimination of novel upright and inverted faces. |
| Functional connectivity fMRI | Song et al., 2015 [75] | 17 DPs, 17 healthy adults, 25 healthy children | Resting state | Core face network’s functional connectivity was disrupted in the DPs. | DPs scored below (3 SD) the normal range on FFT. |
| Functional connectivity fMRI | Zhao et al., 2022 [71] | 64 DPs, 62 HCs | Resting state | Functional connectivity between the FFA and MTG was disrupted in DPs. | DPs demonstrated face-specific impairments during 4 phases of a multiple-stage procedure, while low-level vision, multimodal person recognition, and general object recognition were intact. In the last stage, DPs scored below (1 SD) the normative range on a computer-based O/NFT and FFT. |
| Functional connectivity fMRI (voxel-based global connectivity) | Zhao et al., 2018 [53] | 64 DPs, 62 HCs | Resting state | Both the functional connectivity within the core face network and those between the core face network and extended face network were largely reduced in DPs. Importantly, the r-OFA and r-FFA served as the dysconnectivity hubs within the core face network. In addition, DPs’ r-FFA also showed reduced functional connectivity with the extended face network. This disrupted connectivity was related to DP’s face recognition deficit. | DPs demonstrated face-specific impairments during 4 phases of a multiple-stage procedure, while low-level vision, multimodal person recognition, and general object recognition were intact. In the last stage, DPs scored below (1 SD) the normative range on a computer-based O/NFT and FFT. |
| Structural MRI | Behrmann et al., 2007 [80] | 6 DPs, 12 HCs | None | DPs evinced larger MTG and a significantly smaller aFG compared to HCs. | DPs scored significantly lower than HCs in recognizing familiar faces and making same/different discrimination judgments on unfamiliar faces. DPs were also impaired, although to a lesser extent and with greater variability, on tasks involving nonface stimuli. All DPs performed within the normal range on various low-level visual processing tasks. |
| Structural MRI | Bentin et al., 1999 [81] | 1 DP, 15 HCs | None | DP case showed smaller right temporal lobes compared to HCs. | DP case showed normal visual perception, whereas performance with faces, although within normal range, was lower than HCs. |
| Structural MRI (voxel-based morphometry) | Garrido et al., 2009 [82] | 17 DPs, 18 HCs | None | DPs had reduced grey matter volume in the r-aITG, STS/MTG bilaterally, and in the r-FG and r-aITG compared with HCs. Facial identity task performance correlated with l-STS/MTG and r-FG/ITG gray matter volumes. | DPs reported substantial life-long difficulties with face processing. DPs scored below (2 SD) the normal range on the FFT and the CFMT. DPs did not differ from HCs in IQ scores and low-level perceptual abilities. |
| Structural MRI | Gilaie-Dotan et al., 2009 [61] | 1 DP, 9 HCs | None | No structural abnormalities in the DP case. | The DP case was strongly impaired in recognizing familiar and unfamiliar faces but fairly accurate at recognizing words, familiar places, and buildings. |
| Structural MRI | Van den Stock et al., 2012 [83] | 1 DP, 20 HCs | None | Hypoplasia of the vermis cerebelli. | Low- and mid-level visual perception and object recognition abilities were intact in DP case, whereas face memory and recognition were impaired. |
| Structural MRI | Grossi et al., 2014 [74] | 1 DP, 7 HCs | None | Mild cortical white matter atrophy (amygdala and hippocampal) in the bilateral medial temporal lobe. | The DP case scored borderline on the BFRT and was able to identify the faces’ gender but was not able to distinguish famous from unclear faces and could neither name nor identify celebrities from their photographs on the FFT. The DP case showed mild visual agnosia for living and man-made objects. The general neuropsychological assessment did not reveal impairments. |
| Structural MRI (voxel-based morphometry) | Dinkelacker et al., 2011 [58] | 24 DPs, 25 HCs | None | DPs showed diminished gray matter density in the bilateral lingual gyrus, r-MTG, and l-DLPFC. In most of these areas, gray matter density correlated with memory success. | DPs were impaired in long-term memory for faces and, to a lesser extent, other complex visual stimuli such as buildings. However, their memory for faces with negative valence and their ability to categorize emotional expressions were preserved. |
| Structural MRI (voxel-based morphometry) | Haeger et al., 2021 [77] | 13 DPs, 12 HCs | None | No structural abnormalities in the DP case. | DP diagnosis was based on a complex pattern of features, representing both clinical complaints of long-term memory deficits and compensatory strategies, CFMT score, and family history for some of the DP participants. The CFMT revealed a significant difference in accuracy between DPs and HCs. |
| Protocol/ Outcomes | Reference | Participants | Task Administered during the Protocol | Main Findings | Diagnosis and Behavioral Data |
|---|---|---|---|---|---|
| ERP (N170) | Gilaie-Dotan et al., 2009 [61] | 1 DP, 9 HCs | Passive viewing (faces, watches, and flowers) | No face-selective N170 emerged in the DP subject. | The DP case was strongly impaired at recognizing familiar and unfamiliar faces but fairly accurate at recognizing words, familiar places, and buildings. |
| ERP (N170) | Bentin et al., 1999 [81] | 1 DP, 12 HCs | The matching task for covert recognition (names/faces of politicians vs. movie stars) | No face-selective N170 emerged in the DP subject. | The DP case showed normal visual perception, whereas performance with faces, although within the normal range, was lower than HCs. |
| ERP (N170) | Bentin et al., 2007 [84] | 1 DP, 12 HCs | One-back task (faces, places, and objects) | No face-selective responses emerged in the DP subject. As compared to HCs, the FFA of DPs responded more to objects than faces. No face-specific N170 emerged in the DPs. | The DP case scored lower than HCs in the FFT, CFMT, WFMT (but not words), BFRT, and Boston Naming Test (object recognition). The DP case performed well on the one-back task. |
| ERP (N170) | Burns et al., 2014 [85] | 8 DPs, 11 HCs | “Remember/know” paradigm for recollection and familiarity | Delayed right posterior area’s N170 responses, unexpected frontal responses, and no posterior responses associated with familiarity for faces emerged in DPs. | DPs scored below (2 SD) the normal range on the CFMT and FFT and exhibited significantly worse performances on the “Remember/know” task than HCs. |
| ERP (N170) | Collins et al., 2017 [86] | 7 DPs, 10 HCs | Face/word stimuli (participants were asked to respond if two sequentially presented stimuli were the same or not) | As compared to HCs, DPs did not exhibit the typical N170 hemispheric preference (i.e., left for words and right for faces). | DPs scored below (1.5 SD) the normal range on the CFMT and FFT. DPs performed worse than HCs in detecting the same faces but not the same words. |
| ERP (N170) | DeGutis et al., 2007 [57] | 1 DP, 24 HCs | One-back task (faces, scenes); face classification training | DP’s N170 responses for pictures of faces and watches were comparable, while, during active training, a conspicuous selectivity emerged and resembled that observed in HCs. | The DP case was severely impaired in face recognition and classification, whereas she was within the normal range on the one-back face task and object and word recognition. Following training, the DP case’s performance on face classification task improved. |
| ERP (N170) | Kress & Daum, 2003 [87] | 2 DPs | Classification of known/unknown faces, hand, and houses: hands direction task; face recognition test | Same-amplitude N170 for faces and houses in DPs. | One DP case scored below (2 SD) the normal range at the RMT, while the other one scored within the normal range. Both DPs exhibited poor performances on face recognition, while basic perceptual abilities and facial identity and affect discriminations were intact. |
| ERP (N170) | Lueschow et al., 2015 [88] | 13 DPs, 16 HCs | Motion discrimination task; famous face/building recognition | Face-selective N170 was indistinguishable between DPs and HCs. | DPs scored lower than HCs on the BFRT, the WRMT, the CFMT, and the FFT, while their performances for house recognition were indistinguishable from HCs. |
| ERP (N170) | Minnebusch et al., 2007 [89] | 4 DPs, 55 HCs | Viewing of stimuli from 16 categories, including famous/non-famous faces and various objects | Three out of 4 DPs showed reliable N170 amplitude differences between faces and nonface stimuli. One DP individual showed significantly reduced amplitude differences between faces and nonface objects. | DPs face recognition was heterogeneously impaired with normal basic visual processing and intact with object recognition abilities. |
| ERP (N170) | Németh et al., 2014 [90] | 3 DPs, 20 HCs | Category (face–non face) discrimination task | Reduced DPs’ N170 sensitivity—potentially due to larger noise-elicited N170, rather than smaller face-elicited N170. | DPs showed impaired face recognition on CFMT, whereas object recognition and IQ were in the normal range. |
| ERP (N170) | Towler et al., 2012 [91] | 16 DPs, 16 HCs | One-back task (faces and houses) | No face inversion effect (i.e., enhanced N170 for inverted stimuli) emerged in DPs. | DPs exhibited impaired performances on the CFMT, the FFT, and the O/NFT compared with HCs. There was also evidence for (upright) face perception deficits in the CFPT upright and inverted, while low-level visual perceptual abilities were normal |
| ERP (N170, P1) | Righart & de Gelder, 2007 [92] | 4 DPs, 12 HCs | Orientation–decision task (upright versus inverted) | No configural encoding in 3 out of 4 DPs for faces at the P1component, and for both faces and bodies at the N170 component. | All DPs scored worse than HCs on the BFRT, while 3 out of 4 DPs scored below average at the WFMT. |
| ERP (N250) | [93] | 12 DPs, 12 HCs | Identity/expression-matching task | Attenuated N250 correlating positively with CFMT emerged in DPs. | DPs scored below (2 SD) the normal range at least at 2 out of 3 different diagnostic tests (FFT, CFMT, CFPT) while performing within the normal range on the RMET. In addition, DPs performed worse than controls on the Identity-matching task, while no differences emerged from the expression-matching task. |
| ERP (N250) | [94] | 14 DPs, 14 HCs | Face-matching task | Attenuated N250 emerged in DPs compared to HCs. | DPs scored below (2 SD) the normal range on the FFT, the O/NFT, and the CFMT. The majority of DPs performed within the normal range on the CFPT but worse than HCs on the face-matching task |
| ERP (N250, P600) | Eimer et al., 2012 [95] | 12 DPs, 16 HCs | The task for familiarity judgment of famous and non-famous faces | Occipito-temporal N250 responses emerged in 6 out of 12 DPs. Non-recognized famous faces did not trigger a P600f component in 11 out of 12 DPs. | DPs scored below HCs on the CFMT, CFPT (with only one exception), O/NFT, and in famous and non-famous faces familiarity judgment. |
| ERPs | Parketny et al., 2015 [96] | 10 DPs, 10 HCs | Target identification task | Reliable but delayed N250 and P600f emerged in DPs. | DPS scored below (2 SD) the normal range on the FFT, CFMT, CFPT, and O/NFT. Scores for the target identification task were poorer in DPs compared to HCs. |
| EEG summed amplitude values | Fisher et al., 2020 [97] | 10 DPs, 10 HCs (exp. 1), 12 DPs, 12 HCs (exp. 2) | Oddball discrimination task with upright/inverted faces (exp.1) and faces/cars (exp.2) | Attenuated neural responses (SNS values) to oddball changes emerged in DPs compared to HCs (for both faces and cars). | DPs scored below (2 SD) the normal range on the FFT, the CFMT, and the O/NFT. Performance on the CFPT was more heterogeneous. DPs performed within the normal range on the old/new car memory test. |
| ERPs (source reconstructions) | Olivares et al., 2021 [98] | 1 DP, 14 HCs | The face-feature-matching task with unknown faces, including external (E-) and internal (I-) facial features presented sequentially, then followed by complete matching (correct combination of I- and E-features) or mismatching (different I- and E-features) unfamiliar faces (i.e., targets) | More positive waveforms in matching faces than mismatching ones ~300–500 ms in the E-I condition (i.e., a ‘mismatch effect’) emerged in HCs only. Typical P1-N170 for I-features, but no P2, was found in the DP case; a mismatch effect emerged in the DP patient for the I-E sequence (of shorter duration than that of HCs). Differential topological activations emerged between HCs and DPs: posterior and markedly left-sided (around the OFA) in the DP case, posterior and right-sided in HCs. No P3 responses in response to features were found in the DP case. | The DP case exhibited normal QI and face-specific and associative impairment (i.e., no object processing and/or apperceptive impairments). Performances on the face-feature-matching task revealed no significant differences from HCs but a tendency towards erroneous positive “match” responses in both sequences (significant in the E-I condition). |
| Gamma-band activity (MEG source analysis) | Dobel et al., 2011 [99] | 7 DPs, 7 HCs | Recognition of faces differing in familiarity (famous vs. unknown) and orientation (upright vs. inverted) | No increase in gamma-band activity in the left lateral occipitotemporal gyrus and the left inferior temporal gyrus in response to faces emerged in DPs compared to HCs. | DPs showed normal general visual functions, while performances on the FFT differed significantly from those of HCs. DPs identified significantly less famous faces than HCs in the experimental task for recognition. |
| M170 | Lueschow et al., 2015 [88] | 13 DPs, 16 HCs | Motion detection task, including pictures of faces and houses | Comparable M170 responses to objects emerged in DPs and HCs, while faces elicited prolonged latency and decreased amplitude M170s in DPs. Correlation between face recognition performance and the size of the M170 emerged in HCs but not DPs. | DPs and HCs differed in IQ assessment; however, most of the results from neuropsychological tests were indistinguishable between DPs and HCs. DPs scored significantly lower than HCs on the BFRT, the WRMT, the FFT, and the CFMT, while their performances for houses recognition were analogous to HCs. |
| M170 | Rivolta et al., 2012 [100] | 6 DPs, 11 HCs | Target detection task, including pairs of famous/non-famous faces and places | Greater MEG activity for faces than places (category effect) emerged in the r-LOC and r-FG (i.e., M170 generators) in both HCs and DPs. r-LOC-M170 correlated with holistic/configural face processing, whereas the r-FG-M170 correlated with featural processing. | DPs scored below (2 SD) the normal range on the CFMT and FFT. DPs did not differ from HCs in IQ scores, low-level perceptual abilities, and object recognition, and none of them scored within the autistic range. |
| M170 (neural sources estimation via L2-MNE approach) | Dobel et al., 2008 [101] | 7 DPs, 7 HCs | Recognition of faces differing in familiarity (famous vs. unknown) and orientation (upright vs. inverted) | A bilateral decrease in brain activity in the initial phase of the M170 merged in DPs compared to HCs, followed by an even larger reduction over occipitotemporal areas in the left hemisphere (i.e., lateralization effect), irrespectively of familiarity or orientation of the stimuli. | DPs showed normal general visual functions, while their performances on the FFT differed significantly from those of HCs. DPs identified significantly less famous faces than HCs in the experimental task. |
| M170 + N170 | Harris et al., 2005 [102] | 5 DPs, 8 HCs | Viewing of pictures, including faces, houses, and miscellaneous objects | M170 was not face-selective in 3 of 5 DPs. ERPs in the remaining DPs showed N170s within the same normal range. | Only one DP exhibited impaired performances on visual function tests. All DPs scored 2 SD below the mean on the FFT and between in the O/NFT. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manippa, V.; Palmisano, A.; Ventura, M.; Rivolta, D. The Neural Correlates of Developmental Prosopagnosia: Twenty-Five Years on. Brain Sci. 2023, 13, 1399. https://doi.org/10.3390/brainsci13101399
Manippa V, Palmisano A, Ventura M, Rivolta D. The Neural Correlates of Developmental Prosopagnosia: Twenty-Five Years on. Brain Sciences. 2023; 13(10):1399. https://doi.org/10.3390/brainsci13101399
Chicago/Turabian StyleManippa, Valerio, Annalisa Palmisano, Martina Ventura, and Davide Rivolta. 2023. "The Neural Correlates of Developmental Prosopagnosia: Twenty-Five Years on" Brain Sciences 13, no. 10: 1399. https://doi.org/10.3390/brainsci13101399
APA StyleManippa, V., Palmisano, A., Ventura, M., & Rivolta, D. (2023). The Neural Correlates of Developmental Prosopagnosia: Twenty-Five Years on. Brain Sciences, 13(10), 1399. https://doi.org/10.3390/brainsci13101399





