DaSAM: Disease and Spatial Attention Module-Based Explainable Model for Brain Tumor Detection
Abstract
:1. Introduction
2. Literature Review
2.1. Machine Learning
2.2. Deep Learning
2.3. Pre-Trained Models
2.4. Explainable Artificial Intelligence
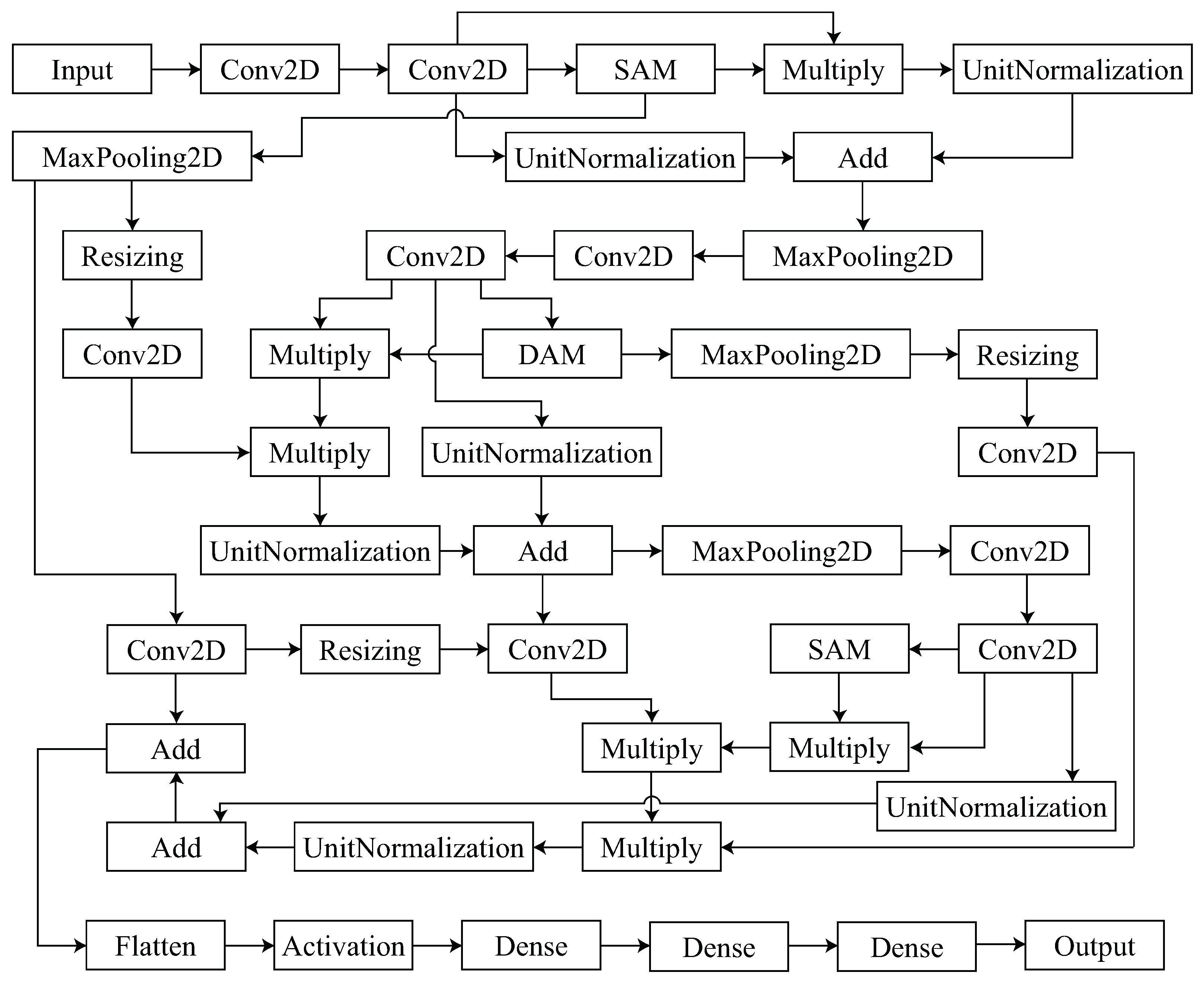
3. Proposed Methodology
3.1. Disease Attention Module
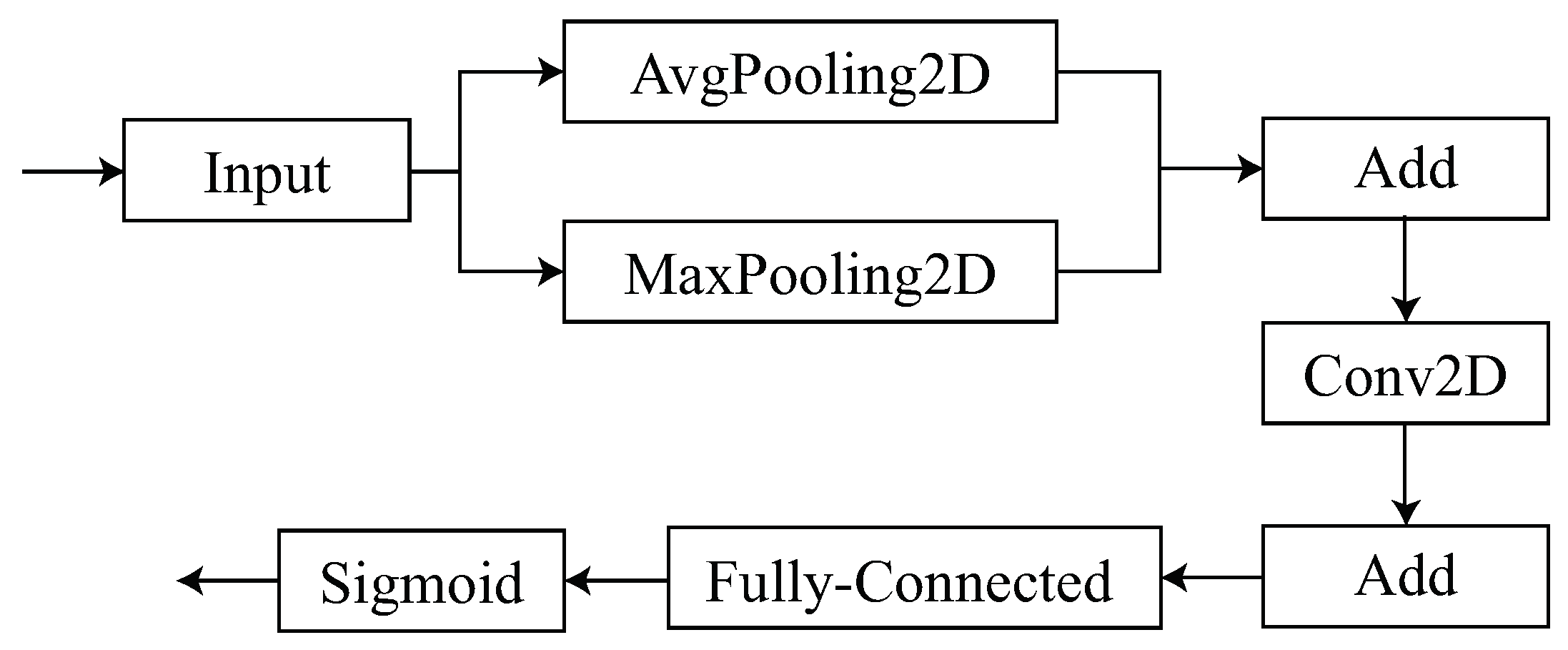
3.2. Spatial Attention Module
3.3. Data Preprocessing and Augmentation
4. Experimental Results
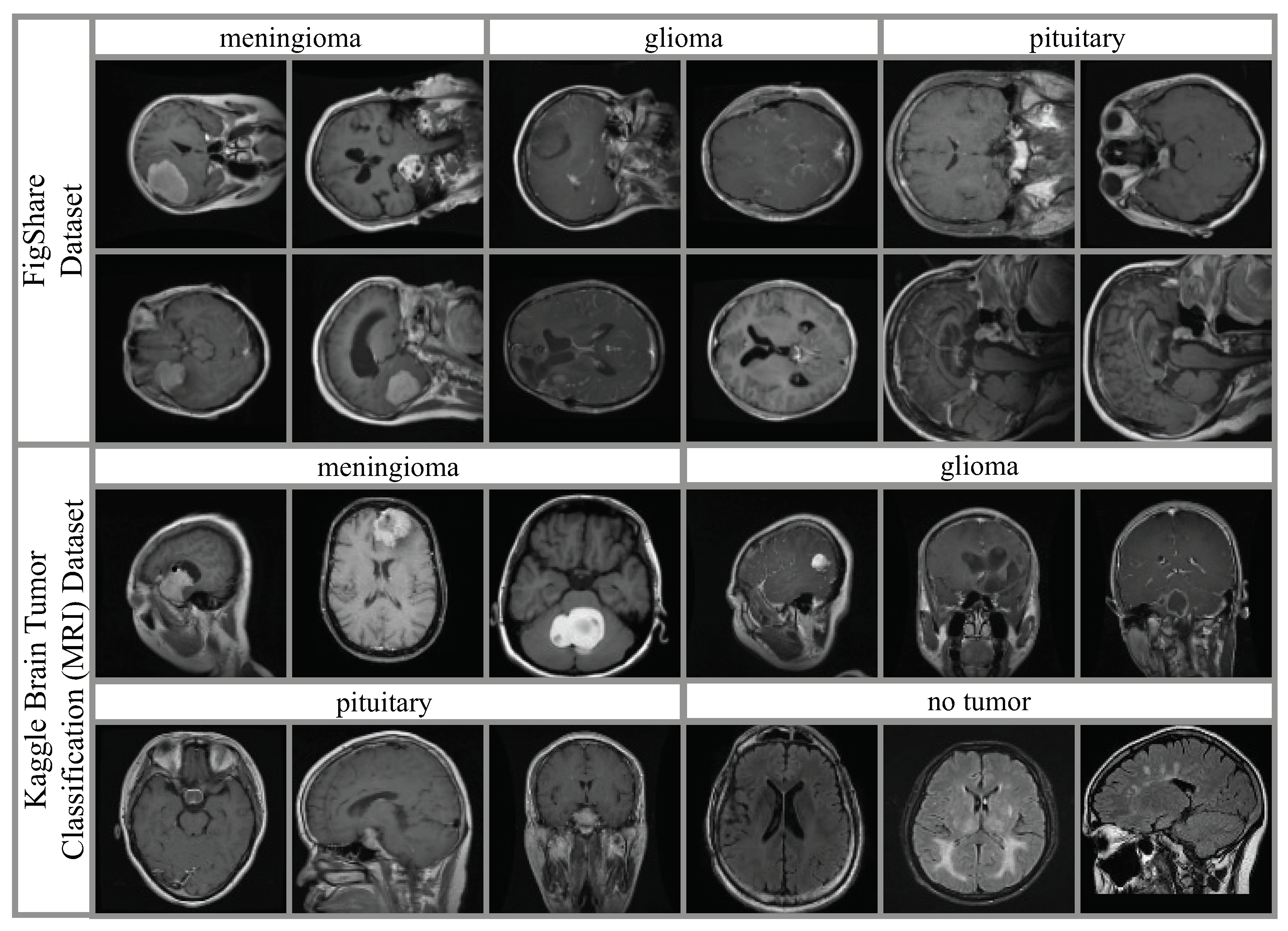
4.1. Datasets and Hyperparameter Settings
4.2. Classification Results
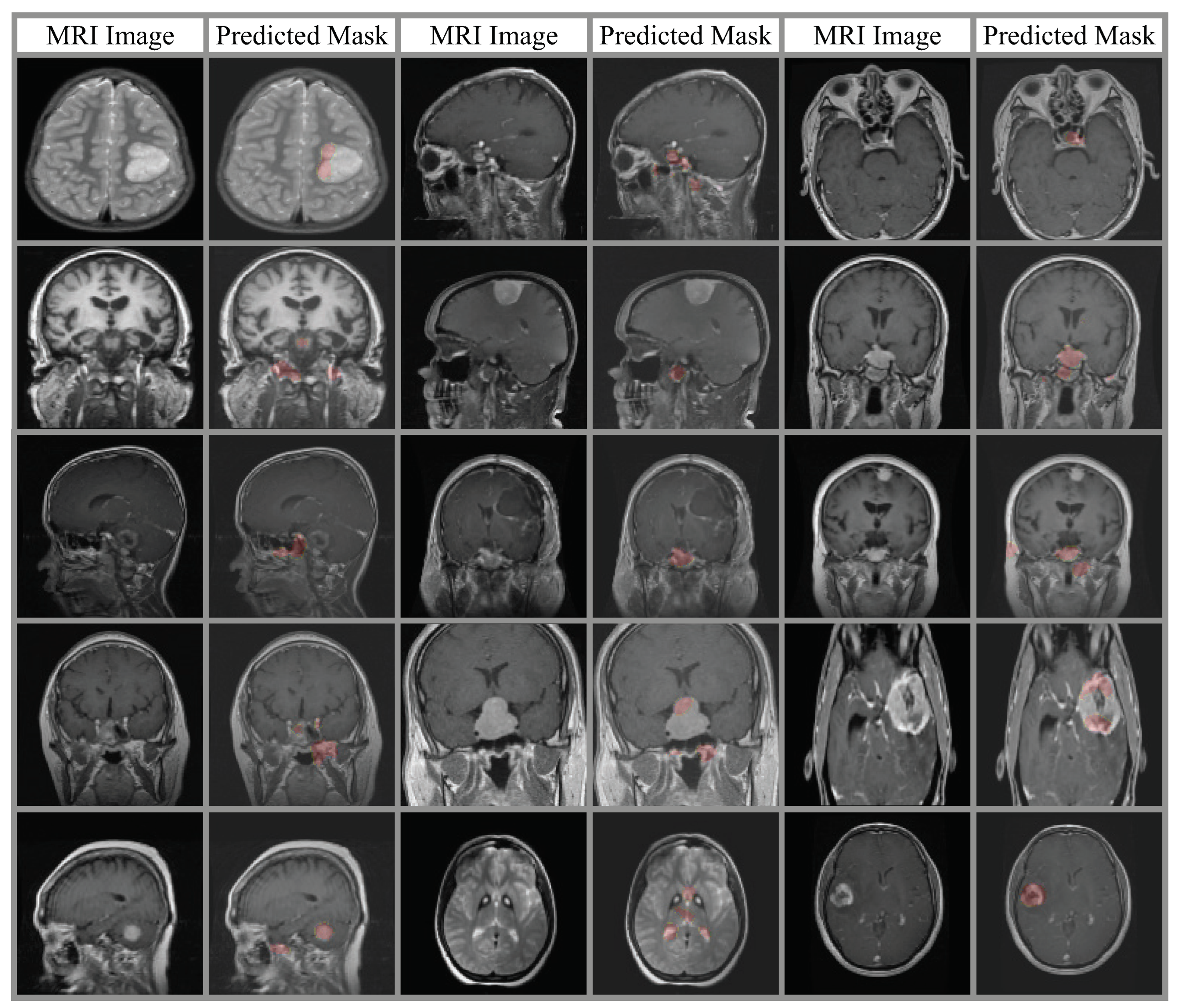
4.3. Ablation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mustaqeem, A.; Javed, A.; Fatima, T. An Efficient Brain Tumor Detection Algorithm Using Watershed & Thresholding Based Segmentation. Int. J. Image Graph. Signal Process. 2012, 4, 34–39. [Google Scholar]
- Meng, Y.; Tang, C.; Yu, J.; Meng, S.; Zhang, W. Exposure to lead increases the risk of meningioma and brain cancer: A meta-analysis. J. Trace Elem. Med. Biol. 2020, 60, 126474. [Google Scholar] [CrossRef] [PubMed]
- El-Dahshan, E.-S.A.; Mohsen, H.M.; Revett, K.; Salem, A.-B.M. Computer-aided diagnosis of human brain tumor through MRI: A survey and a new algorithm. Expert Syst. Appl. 2014, 41, 5526–5545. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Wang, J.; Wu, F.; Liu, T.; Pan, Y. A Survey of MRI-Based Brain Tumor Segmentation Methods. Tsinghua Sci. Technol. 2014, 19, 578–595. [Google Scholar]
- Maqsood, S.; Damasevicius, R.; Shah, F.M. An Efficient Approach for the Detection of Brain Tumor Using Fuzzy Logic and U-NET CNN Classification. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2021; Volume 12953 LNCS, pp. 105–118. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Battineni, G.; Sagaro, G.G.; Chinatalapudi, N.; Amenta, F. Applications of Machine Learning Predictive Models in the Chronic Disease Diagnosis. J. Pers. Med. 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zuo, C.; Qian, J.; Feng, S.; Yin, W.; Li, Y.; Fan, P.; Han, J.; Qian, K.; Chen, Q. Deep learning in optical metrology: A review. Light Sci. Appl. 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Ren, L.; Cai, J. Clinical implementation of AI technologies will require interpretable AI models. Med. Phys. 2020, 47, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, Y.; Lund, J. Applications of Explainable Artificial Intelligence in Diagnosis and Surgery. Diagnostics 2022, 12, 237. [Google Scholar] [CrossRef]
- Hassija, V.; Chamola, V.; Mahapatra, A.; Singal, A.; Goel, D.; Huang, K.; Scardapane, S.; Spinelli, I.; Mahmud, M.; Hussain, A. Interpreting Black-Box Models: A Review on Explainable Artificial Intelligence. Cognit. Comput. 2023, 16, 45–74. [Google Scholar] [CrossRef]
- Naik, J.; Patel, S. Tumor detection and classification using decision tree in brain MRI. Int. J. Comput. Sci. Netw. Secur. 2014, 14, 87. [Google Scholar]
- Shil, S.; Polly, F.; Hossain, M.A.; Ifthekhar, M.S.; Uddin, M.N.; Jang, Y.M. An improved brain tumor detection and classification mechanism. In Proceedings of the 2017 International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Republic of Korea, 18–20 October 2017; pp. 54–57. [Google Scholar]
- Mathew, A.R.; Anto, P.B. Tumor detection and classification of MRI brain image using wavelet transform and SVM. In Proceedings of the IEEE 2017 International Conference on Signal Processing and Communication (ICSPC), Coimbatore, India, 28–29 July 2017; pp. 75–78. [Google Scholar]
- Singh, D.; Kaur, K. Classification of abnormalities in brain MRI images using GLCM, PCA and SVM. Int. J. Eng. Adv. Technol. 2012, 1, 243–248. [Google Scholar]
- Amin, J.; Sharif, M.; Yasmin, M.; Fernandes, S.L. A distinctive approach in brain tumor detection and classification using MRI. Pattern Recognit. Lett. 2020, 139, 118–127. [Google Scholar] [CrossRef]
- Ramteke, R.; Monali, Y.K. Automatic medical image classification and abnormality detection using K-Nearest Neighbour. Int. J. Adv. Comput. Res. 2012, 2, 190–196. [Google Scholar]
- Rajinikanth, V.; Kadry, S.; Nam, Y. Convolutional-Neural-Network Assisted Segmentation and SVM Classification of Brain Tumor in Clinical MRI Slices. Inf. Tech. Control 2021, 50, 342–356. [Google Scholar] [CrossRef]
- Kurdi, S.Z.; Ali, M.H.; Jaber, M.M.; Saba, T.; Rehman, A.; Damaševičius, R. Brain Tumor Classification Using Meta-Heuristic Optimized Convolutional Neural Networks. J. Pers. Med. 2023, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, A.; Alhaisoni, M.; Alqahtani, A.; Alsubai, S.; Alharbi, M.; Malik, N.; Damaševičius, R. Multimodal brain tumor detection and classification using deep saliency map and improved dragonfly optimization algorithm. Int. J. Imaging Syst. Technol. 2023, 33, 572–587. [Google Scholar] [CrossRef]
- Badjie, B.; Ülker, E.D. A Deep Transfer Learning Based Architecture for Brain Tumor Classification Using MR Images. Inform. Tech. Control 2022, 51, 332–344. [Google Scholar] [CrossRef]
- Zheng, Q.; Saponara, S.; Tian, X.; Yu, Z.; Elhanashi, A.; Yu, R. A real-time constellation image classification method of wireless communication signals based on the lightweight network MobileViT. Cogn. Neurodynamics 2024, 18, 659–671. [Google Scholar] [CrossRef]
- Pereira, S.; Meier, R.; Alves, V.; Reyes, M.; Silva, C.A. Automatic brain tumor grading from MRI data using convolutional neural networks and quality assessment. In Understanding and Interpreting Machine Learning in Medical Image Computing Applications; Springer: Cham, Switzerland, 2018; pp. 106–114. [Google Scholar]
- Seetha, J.; Raja, S. Brain tumor classification using convolutional neural networks. Biomed. Pharmacol. J. 2018, 11, 1457–1461. [Google Scholar] [CrossRef]
- Bhanothu, Y.; Kamalakannan, A.; Rajamanickam, G. Detection and classification of brain tumor in MRI images using deep convolutional network. In Proceedings of the 2020 6th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 6–7 March 2020; pp. 248–252. [Google Scholar]
- Badža, M.M.; Barjaktarović, M.Č. Classification of brain tumors from mri images using a convolutional neural network. Appl. Sci. 2020, 10, 1999. [Google Scholar] [CrossRef]
- Das, S.; Aranya, O.R.R.; Labiba, N.N. Brain Tumor Classification Using Convolutional Neural Network. In Proceedings of the 2019 1st International Conference on Advances in Science, Engineering and Robotics Technology (ICASERT), Dhaka, Bangladesh, 3–5 May 2019; pp. 1–5. [Google Scholar]
- Afshar, P.; Mohammadi, A.; Plataniotis, K.N. Brain Tumor Type Classification via Capsule Networks. In Proceedings of the 2018 25th IEEE International Conference on Image Processing (ICIP), Athens, Greece, 7–10 October 2018; pp. 3129–3133. [Google Scholar]
- Khan, H.A.; Jue, W.; Mushtaq, M.; Mushtaq, M.U. Brain tumor classification in MRI image using convolutional neural network. Math. Biosci. Eng. 2020, 17, 6203–6216. [Google Scholar] [CrossRef]
- Swati, Z.N.K.; Zhao, Q.; Kabir, M.; Ali, F.; Ali, Z.; Ahmed, S.; Lu, J. Contentbased brain tumor retrieval for mr images using transfer learning. IEEE Access 2019, 7, 809–817. [Google Scholar] [CrossRef]
- Deepak, S.; Ameer, P.M. Brain Tumor Classification using Deep CNN features via transfer learning. Comput. Biol. Med. 2019, 111, 103345. [Google Scholar] [CrossRef] [PubMed]
- Chelghoum, R.; Ikhlef, A.; Hameurlaine, A.; Jacquir, S. Transfer learning using convolutional neural network architectures for brain tumor classification from MRI images. In Proceedings of the IFIP International Conference on Artificial Intelligence Applications and Innovations, Neos Marmaras, Greece, 5–7 June 2020; pp. 189–200. [Google Scholar]
- Mehrotra, R.; Ansari, M.A.; Agrawal, R.; Anand, R.S. A transfer learning approach for AI-based classification of brain tumors. Mach. Learn. Appl. 2020, 2, 100003. [Google Scholar] [CrossRef]
- Natekar, P.; Kori, A.; Krishnamurthi, G. Demystifying brain tumor segmentation networks: Interpretability and uncertainty analysis. Front. Comput. Neurosci. 2020, 14, 6. [Google Scholar] [CrossRef]
- Esmaeili, M.; Vettukattil, R.; Banitalebi, H.; Krogh, N.R.; Geitung, J.T. Explainable artificial intelligence for human-machine interaction in brain tumor localization. J. Pers. Med. 2021, 11, 1213. [Google Scholar] [CrossRef]
- Windisch, P.; Weber, P.; Fürweger, C.; Ehret, F.; Kufeld, M.; Zwahlen, D.; Muacevic, A. Implementation of model explainability for a basic brain tumor detection using convolutional neural networks on MRI slices. Neuroradiology 2020, 62, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Pikulkaew, K. Enhancing Brain Tumor Detection with Gradient-Weighted Class Activation Mapping and Deep Learning Techniques. In Proceedings of the 2023 20th International Joint Conference on Computer Science and Software Engineering (JCSSE), Phitsanulok, Thailand, 28 June 2023; pp. 339–344. [Google Scholar]
- Saleem, H.; Shahid, A.R.; Raza, B. Visual interpretability in 3D brain tumor segmentation network. Comput. Biol. Med. 2021, 133, 104410. [Google Scholar] [CrossRef]
- Zeineldin, R.A.; Karar, M.E.; Elshaer, Z.; Coburger, J.; Wirtz, C.R.; Burgert, O.; Mathis-Ullrich, F. Explainability of deep neural networks for MRI analysis of brain tumors. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Brain Tumor Classification (MRI). Available online: https://www.kaggle.com/datasets/sartajbhuvaji/brain-tumor-classification-mri (accessed on 1 March 2024).
- Figshare Brain Tumor Dataset. Available online: https://figshare.com/articles/dataset/brain-tumor-dataset/1512427 (accessed on 1 March 2024).
- Lamrani, D.; Cherradi, B.; El Gannour, O.; Bouqentar, M.A.; Bahatti, L. Brain tumor detection using MRI images and convolutional neural network. Int. J. Adv. Comput. Sci. Appl. 2022, 13, 452–460. [Google Scholar] [CrossRef]
- Minarno, A.E.; Mandiri, M.H.C.; Munarko, Y.; Hariyady, H. Convolutional neural network with hyperparameter tuning for brain tumor classification. Kinetik Game Technol. Inf. Syst. Comput. Netw. Comput. Electron. Control 2021, 6, 127–132. [Google Scholar]
- Saxena, P.; Maheshwari, A.; Maheshwari, S. Predictive modeling of brain tumor: A Deep learning approach. In Innovations in Computational Intelligence and Computer Vision; Springer: Singapore, 2021; pp. 275–285. [Google Scholar]
- Rahman, T.; Islam, M.S. MRI brain tumor classification using deep convolutional neural network. In Proceedings of the 2022 3rd International Conference on Innovations in Science, Engineering and Technology (ICISET), Chittagong, Bangladesh, 26–27 February 2022; pp. 451–456. [Google Scholar]
- Rahman, T.; Islam, M.S. MRI brain tumor detection and classification using parallel deep convolutional neural networks. Meas. Sens. 2023, 26, 100694. [Google Scholar] [CrossRef]


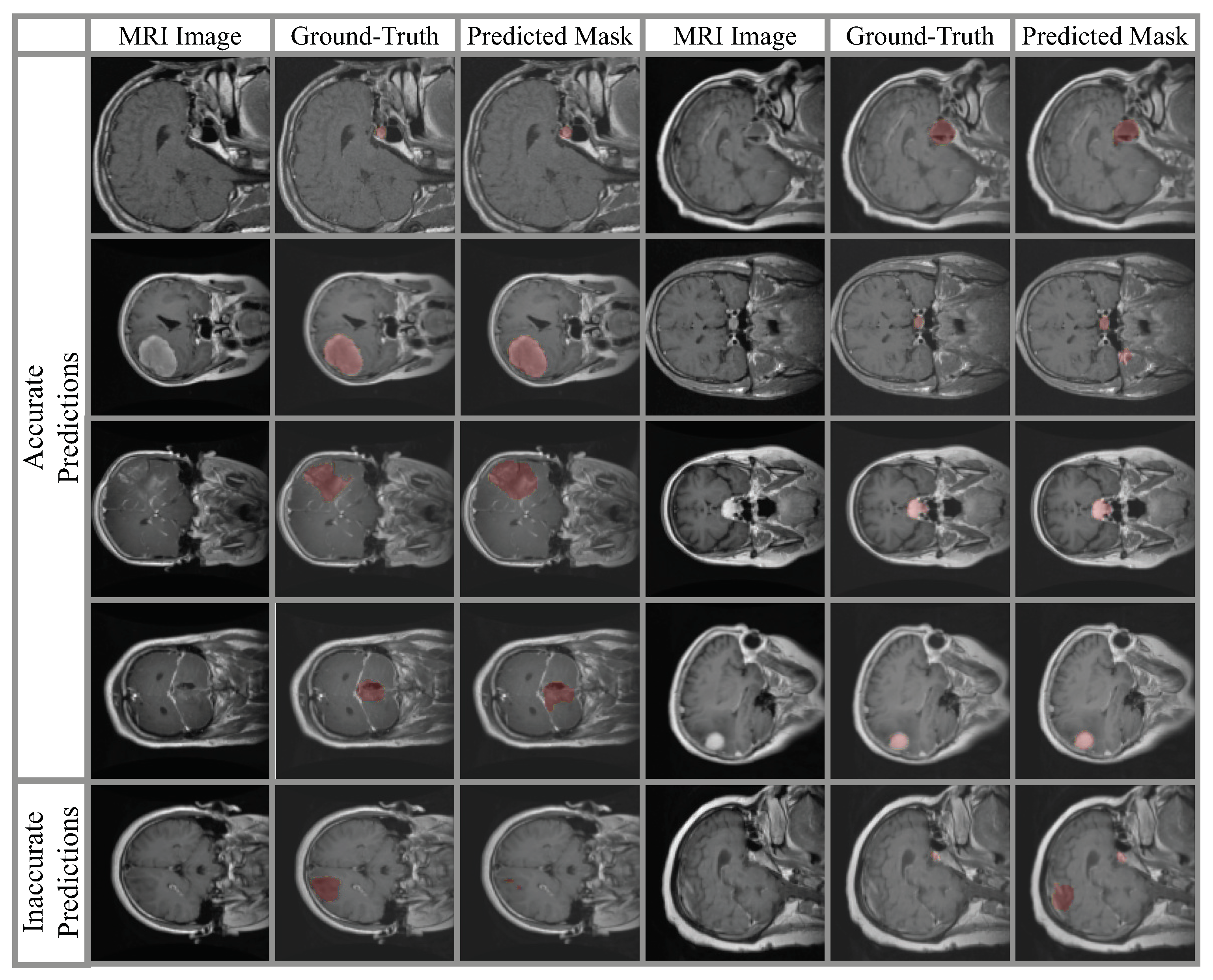
| Parameters | Value |
|---|---|
| Epochs | 100 |
| Batch Size | 32 |
| Epsilon | 0.1 |
| Optimizer | Adam |
| Learning Rate | 0.01 |
| Initial Class Weights | Figshare: 0: 1.44, 1: 0.72, 2: 1.09 |
| Kaggle: 0: 0.37, 1: 0.85, 2: 1.37, 3: 1.98 | |
| Early Stopping | Monitor = Validation Loss, Patience = 20, Minimum Change = 0.001 |
| Class | Figshare Dataset | Kaggle Dataset | ||||||
|---|---|---|---|---|---|---|---|---|
| P (%) | R (%) | F (%) | A (%) | P (%) | R (%) | F (%) | A (%) | |
| M | 97 | 100 | 97 | 99 | 92 | 97 | 95 | 96 |
| G | 100 | 96 | 98 | 98 | 94 | 96 | ||
| P | 99 | 100 | 99 | 93 | 97 | 94 | ||
| N | - | - | - | 94 | 94 | 95 | ||
| Macro Average | 98 | 99 | 98 | 95 | 97 | 94 | ||
| Weighted Average | 98 | 98 | 98 | 95 | 96 | 94 | ||
| Classes | P (%) | R (%) | F (%) | A (%) |
|---|---|---|---|---|
| M | 83 | 80 | 85 | 85 |
| G | 80 | 81 | 79 | |
| P | 78 | 85 | 83 | |
| Macro Average | 81 | 83 | 82 | |
| Weighted Average | 80 | 81 | 84 |
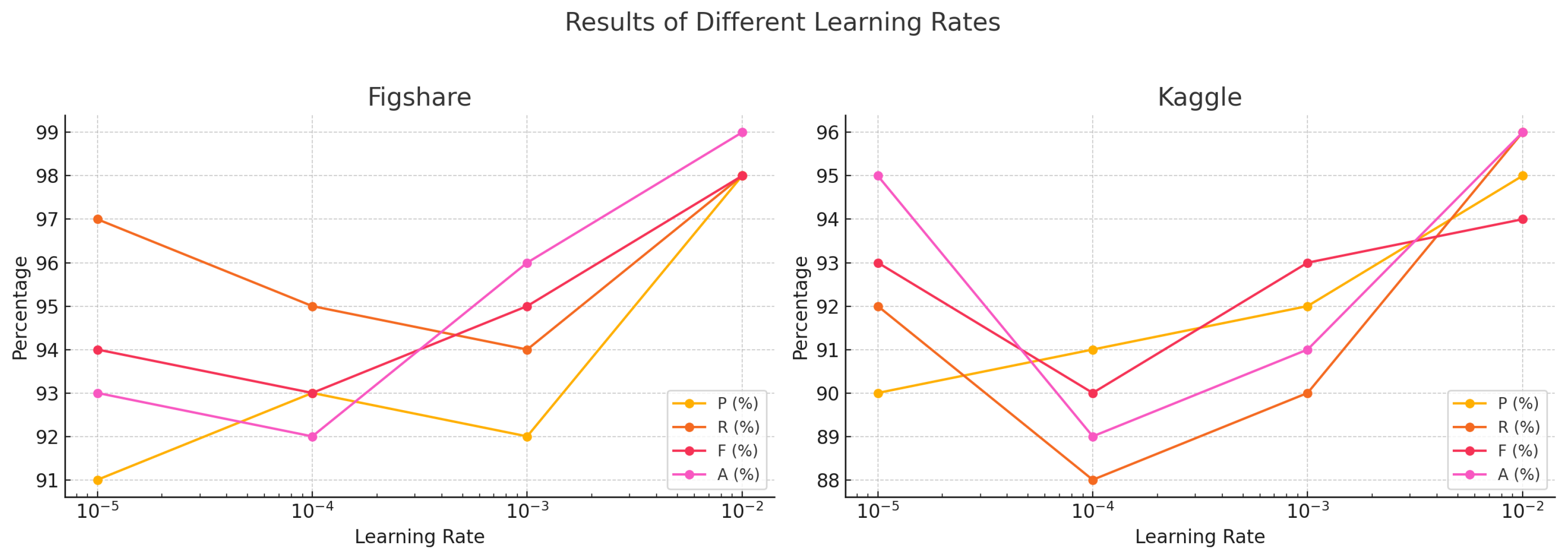
| Results of Different Learning Rates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Learning Rate | Figshare | Kaggle | ||||||
| P (%) | R (%) | F (%) | A (%) | P (%) | R (%) | F (%) | A (%) | |
| 0.01 | 98 | 98 | 98 | 99 | 95 | 96 | 94 | 96 |
| 0.001 | 92 | 94 | 95 | 96 | 92 | 90 | 93 | 91 |
| 0.0001 | 93 | 95 | 93 | 92 | 91 | 88 | 90 | 89 |
| 0.00001 | 91 | 97 | 94 | 93 | 90 | 92 | 93 | 95 |
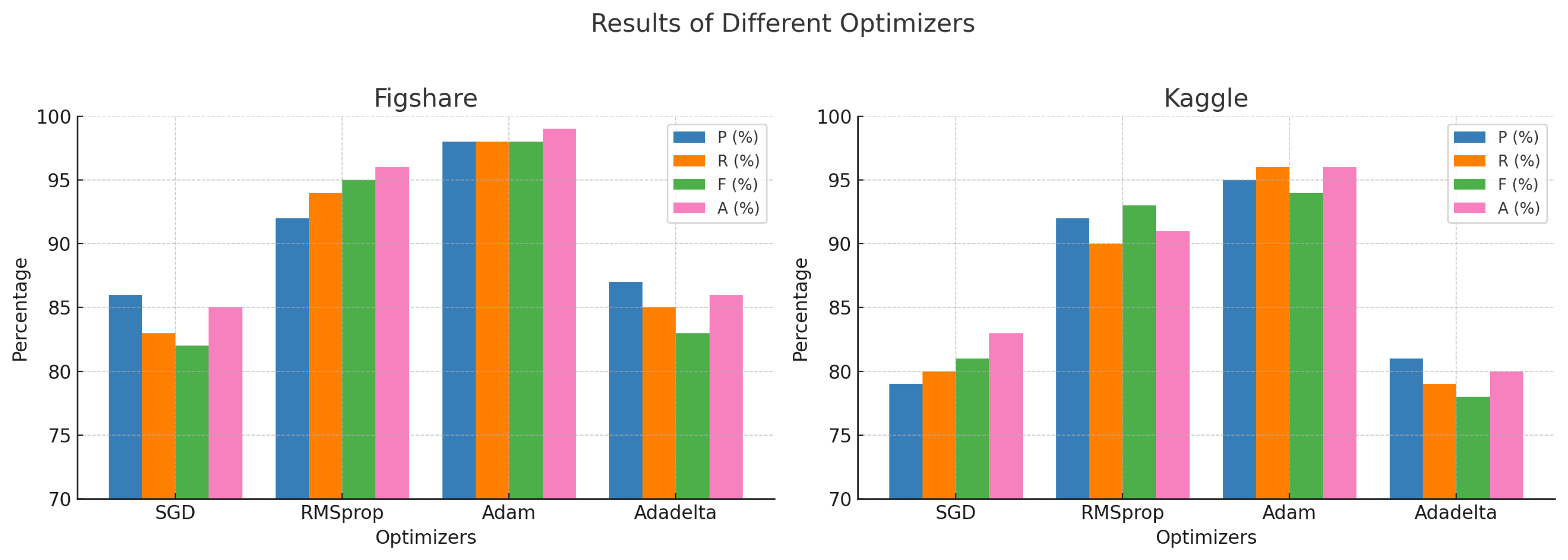
| Results of Different Optimizers | ||||||||
| Optimizers | Figshare | Kaggle | ||||||
| P (% | R (%) | F (%) | A (%) | P (%) | R (%) | F (%) | A (%) | |
| SGD | 86 | 83 | 82 | 85 | 79 | 80 | 81 | 83 |
| RMSprop | 92 | 94 | 95 | 96 | 92 | 90 | 93 | 91 |
| Adam | 98 | 98 | 98 | 99 | 95 | 96 | 94 | 96 |
| Adadelta | 87 | 85 | 83 | 86 | 81 | 79 | 78 | 80 |
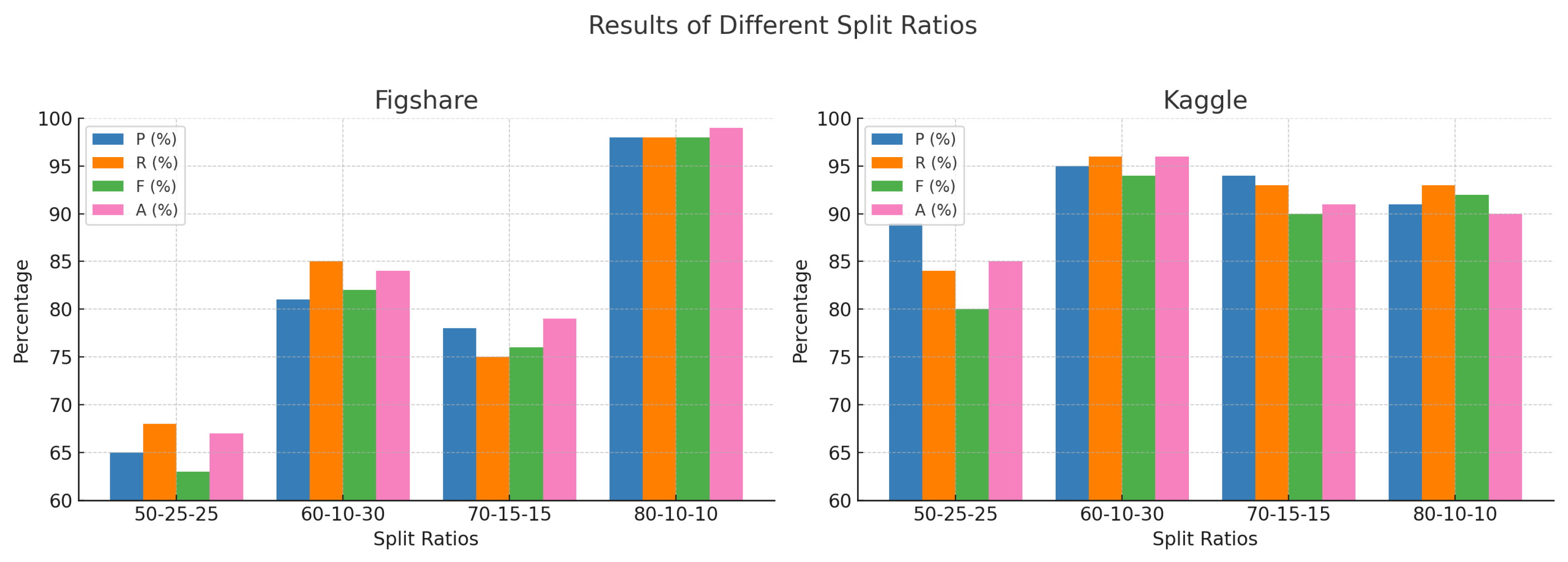
| Results of Different Split Ratio | ||||||||
| Split Ratio | Figshare | Kaggle | ||||||
| P (%) | R (%) | F (%) | A (%) | P (%) | R (%) | F (%) | A (%) | |
| 50-25-25 | 65 | 68 | 63 | 67 | 89 | 84 | 80 | 85 |
| 60-10-30 | 81 | 85 | 82 | 84 | 95 | 96 | 94 | 96 |
| 70-15-15 | 78 | 75 | 76 | 79 | 94 | 93 | 90 | 91 |
| 80-10-10 | 98 | 98 | 98 | 99 | 91 | 93 | 92 | 90 |
| Model | Dataset | Accuracy |
|---|---|---|
| CNN [43] | Figshare | 96% |
| Binary Dataset | 94% | |
| CNN with Pre-processing [44] | Kaggle Dataset | 96% |
| Transfer learning-based CNN [45] | Figshare | 94% |
| Binary Dataset | 95% | |
| CNN with Pre-processing [46] | Figshare | 96% |
| Kaggle Dataset | 94% | |
| CNN with Pre-processing [47] | Figshare | 98% |
| Kaggle Dataset | 98% | |
| Proposed Model | Figshare | 99% |
| Kaggle Dataset | 96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tehsin, S.; Nasir, I.M.; Damaševičius, R.; Maskeliūnas, R. DaSAM: Disease and Spatial Attention Module-Based Explainable Model for Brain Tumor Detection. Big Data Cogn. Comput. 2024, 8, 97. https://doi.org/10.3390/bdcc8090097
Tehsin S, Nasir IM, Damaševičius R, Maskeliūnas R. DaSAM: Disease and Spatial Attention Module-Based Explainable Model for Brain Tumor Detection. Big Data and Cognitive Computing. 2024; 8(9):97. https://doi.org/10.3390/bdcc8090097
Chicago/Turabian StyleTehsin, Sara, Inzamam Mashood Nasir, Robertas Damaševičius, and Rytis Maskeliūnas. 2024. "DaSAM: Disease and Spatial Attention Module-Based Explainable Model for Brain Tumor Detection" Big Data and Cognitive Computing 8, no. 9: 97. https://doi.org/10.3390/bdcc8090097







