Effects of Nitrogen Fertilization on Weed Flora and Productivity of Soybean [Glycine max (L.) Merr.] Crop
Abstract
:1. Introduction
2. Materials and Methods
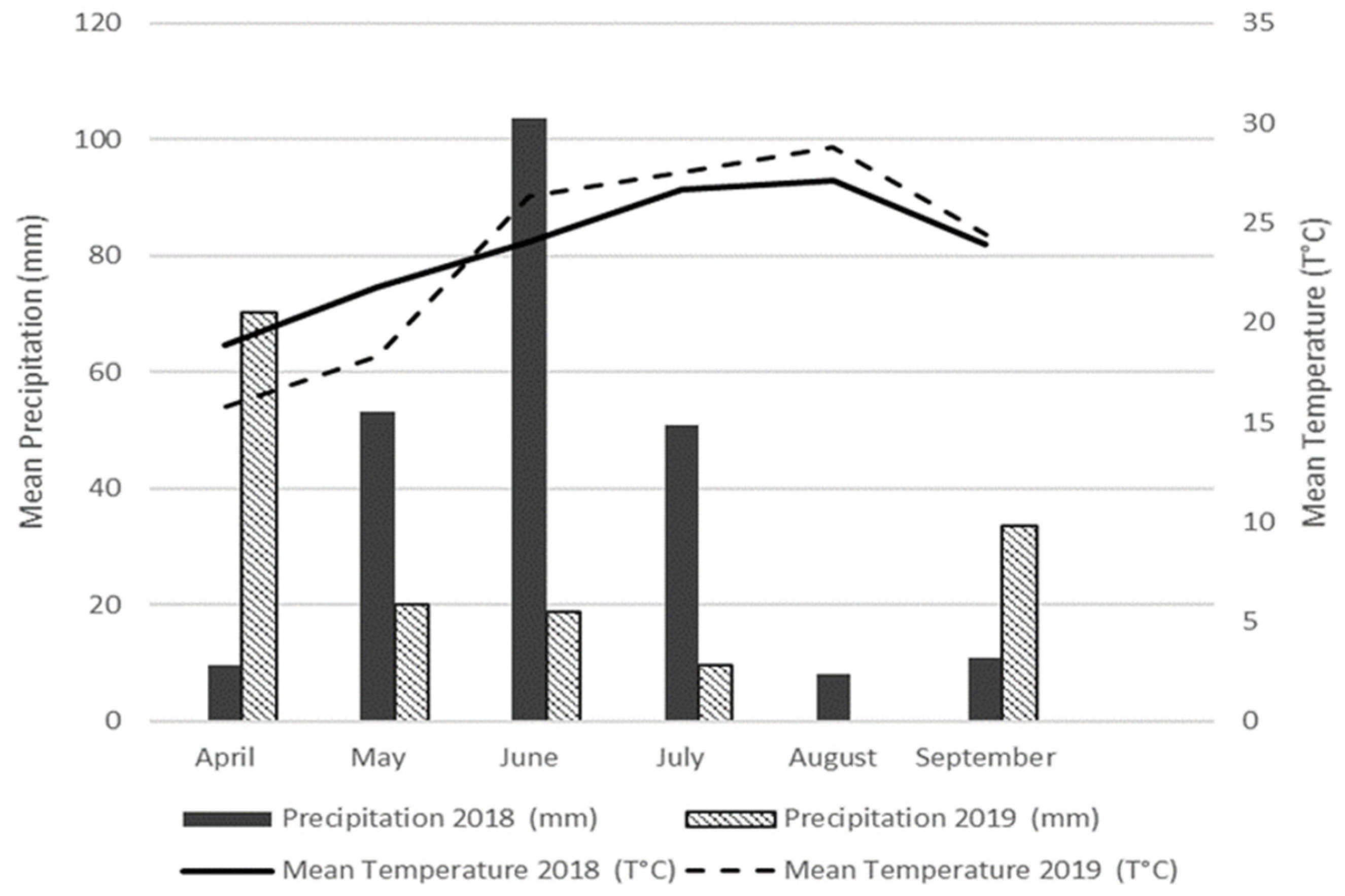
2.1. Study Area
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Soybean Growth Traits and Yield
3.2. Weed Flora and Weed Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Grassini, P.; Cafaro La Menza, N.; Rattalino Edreira, I.G.; Monzón, J.P.; Tenorio, A.F.; Specht, E.J. Soybean. In Crop Physiology Case Histories for Major Crops, 1st ed.; Sadras, O.V., Calderini, F.D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 282–319. [Google Scholar]
- Hartman, G.L.; West, E.D.; Herman, T.K. Crops that feed the World 2. Soybean–worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011, 3, 5–17. [Google Scholar] [CrossRef]
- Hellal, F.A.; Abdelhamid, M.T. Nutrient management practices for enhancing soybean (Glycine max L.) production. Acta Biol. Colomb. 2013, 18, 239–250. [Google Scholar]
- Pagano, M.C.; Miransari, M. The importance of soybean production worldwide. In Abiotic and Biotic Stresses in Soy-Bean Production, 1st ed.; Miransari, M., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, pp. 1–26. [Google Scholar]
- Keyser, H.H.; Li, F. Potential for increasing biological nitrogen fixation in soybean. In Biological Nitrogen Fixation for Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 1992; pp. 119–135. [Google Scholar]
- Zimmer, S.; Messmer, M.; Haase, T.; Piepho, H.P.; Mindermann, A.; Schulz, H.; Habekuß, A.; Ordon, F.; Wilbois, K.P.; Heß, J. Effects of soybean variety and Bradyrhizobium strains on yield, protein content and biological nitrogen fixation under cool growing conditions in Germany. Eur. J. Agron. 2016, 72, 38–46. [Google Scholar] [CrossRef]
- Albuquerque, T.M.; Ortez, O.A.; Carmona, G.I.; Ciampitti, I.A. Soybean: Evaluation of inoculation. Kans. Agric. Exp. Stn. Res. Rep. 2017, 6, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of rhizobium-soybean symbiosis and nitrogen fixation under drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef] [Green Version]
- Zapata, F.; Danso, S.K.A.; Hardarson, G.; Fried, M. Time Course of Nitrogen Fixation in Field-Grown Soybean Using Nitrogen-15 Methodology. Agron. J. 1987, 79, 172–176. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 2015, 6, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Leggett, M.; Diaz-Zorita, M.; Koivunen, M.; Bowman, R.; Pesek, R.; Stevenson, C.; Leister, T. Soybean response to inoculation with Bradyrhizobium japonicum in the United States and Argentina. Agron. J. 2017, 109, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- Kumawat, S.M.; Dhakar, L.L.; Maliwal, P.L. Effect of irrigation regimes and nitrogen on yield, oil content and nutrient uptake of soybean (Glycine max (L.) Merrill). Indian J. Agron. 2000, 45, 361–366. [Google Scholar]
- Chang, W.S.; Lee, H.I.; Hungria, M. Soybean production in the Americas. In Principles of Plant-Microbe Interactions; Springer: Cham, Switzerland, 2015; pp. 393–400. [Google Scholar]
- Griffith, W.K. Satisfying the nutritional requirements of established legumes. Forage Fertil. 1974, 147–169. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Mu, X.; Zhao, G.; Gao, P.; Sun, W. Effects of different fertilization regimes on crop yield and soil water use efficiency of millet and soybean. Sustainability 2020, 12, 4125. [Google Scholar] [CrossRef]
- Wood, C.W.; Torbert, H.A.; Weaver, D.B. Nitrogen fertilizer effects on soybean growth, yield, and seed composition. J. Prod. Agric. 1993, 6, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Salvagiotti, F.; Cassman, K.; Specht, J.; Walters, D.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res. 2008, 108, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Khaledian, M.S.; Mohammadi, K.; Javaheri, M. Grain yield and yield components of soybean affected by integrated fertilization methods. Int. J. Agric. For. 2014, 4, 1–3. [Google Scholar]
- Tamagno, S.; Sadras, V.O.; Haegele, J.W.; Armstrong, P.R.; Ciampitti, I.A. Interplay between nitrogen fertilizer and biological nitrogen fixation in soybean: Implications on seed yield and biomass allocation. Sci. Rep. 2018, 8, 17502. [Google Scholar] [CrossRef] [Green Version]
- Folina, A.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Katsenios, N.; Efthimiadou, A.; Travlos, I.S.; Roussis, I.; Darawsheh, M.K.; Papastylianou, P.; et al. Evaluation of Various Nitrogen Indices in N-Fertilizers with Inhibitors in Field Crops: A Review. Agronomy 2021, 11, 418. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31. [Google Scholar] [CrossRef]
- Samseemoung, G.; Soni, P.; Jayasuriya, H.P.; Salokhe, V.M. Application of low altitude remote sensing (LARS) platform for monitoring crop growth and weed infestation in a soybean plantation. Precis. Agric. 2012, 13, 611–627. [Google Scholar] [CrossRef]
- Fickett, N.D.; Boerboom, C.M.; Stoltenberg, D.E. Soybean yield loss potential associated with early-season weed competition across 64 site-years. Weed Sci. 2013, 61, 500–507. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Datta, A. The critical period for weed control: Revisiting data analysis. Weed Sci. 2015, 63, 188–202. [Google Scholar] [CrossRef] [Green Version]
- Travlos, I.; Tataridas, A.; Kanatas, P.; Kakabouki, I.; Papastylianou, P. Weed management in soybean with a special focus on the control of purple nutsedge (Cyperus rotundus). Agron. Res. 2020, 18, 1–8. [Google Scholar]
- Werner, E.L.; Curran, W.S.; Lingenfelter, D.D. Management of eastern black nightshade in agronomic crops: An integrated approach. Agron. Facts 2014, 58, 1–6. [Google Scholar]
- Sweeney, A.E.; Renner, K.A.; Laboski, C.; Davis, A. Effect of fertilizer nitrogen on weed emergence and growth. Weed Sci. 2008, 56, 714–721. [Google Scholar] [CrossRef]
- Kakabouki, I.; Karkanis, A.; Travlos, I.S.; Hela, D.; Papastylianou, P.; Wu, H.; Chachalis, D.; Sestras, R.; Bilalis, D. Weed flora and seed yield in quinoa crop (Chenopodium quinoa Willd.) as affected by tillage systems and fertilization practices. Int. J. Pest Manag. 2015, 61, 228–234. [Google Scholar] [CrossRef]
- Dew, D.A. An index of competition for estimating crop loss due to weeds. Can. J. Plant Sci. 1972, 52, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.S.; Kumar, S. Research Techniques in Agronomy; Department of Agronomy, College of Agriculture, CSK Himachal Pradesh Krishi Vishvavidyalaya: Palampur, India, 2014; p. 64. [Google Scholar]
- Garko, M.S.; Yawale, M.A.; Gaya, U.H.; Mohammed, I.B.; Bello, T.T. Weed persistence, crop resistance and phytotonic effects of herbicides in maize (Zea mays) production under different weed control method and poultry manure in Kano State Nigeria. J. Biol. Agric. Healthc. 2020, 10, 11–17. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Pielou, E. An Introduction to Mathematical Ecology; Wiley Interscience: New York, NY, USA, 1969. [Google Scholar]
- Margalef, R. Information theory in ecology. Gen. Syst. Yearb. 1958, 3, 36–71. [Google Scholar]
- Travlos, I.S.; Cheimona, N.; Roussis, I.; Bilalis, D.J. Weed-species abundance and diversity indices in relation to tillage systems and fertilization. Front. Environ. Sci. 2018, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dhaka, A.K.; Kumar, S.; Singh, S.; Punia, S.S. Weed management indices as affected by different weed control treatments in pigeon pea [Cajanus cajan (L.) Millsp.]. J. Pharmacogn. Phytochem. 2019, 8, 3490–3494. [Google Scholar]
- Martey, E.; Goldsmith, P. Heterogeneous demand for soybean quality. Afr. J. Agric. Resour. Econ. 2020, 15, 27–50. [Google Scholar] [CrossRef]
- 2012–2013 Lime and Nutrient Recommendations, University of Kentucky, College of Agriculture. Available online: https://uknowledge.uky.edu/cgi/viewcontent.cgi?article=1024&context=anr_reports (accessed on 14 April 2022).
- Ribeiro, V.H.V.; Maia, L.S.G.; Arneson, N.J.; Oliveira, M.C.; Read, H.W.; Ané, J.M.; Santos, B.J.; Werle, R. Influence of PRE-emergence herbicides on soybean development, root nodulation and symbiotic nitrogen fixation. Crop Prot. 2021, 144, 105576. [Google Scholar] [CrossRef]
- Reddy, K.N.; Zablotowicz, R.M. Glyphosate-resistant soybean response to various salts of glyphosate and glyphosate accumulation in soybean nodules. Weed Sci. 2003, 51, 496–502. [Google Scholar] [CrossRef]
- Bohm, G.M.; Alves, B.J.; Urquiaga, S.; Boddey, R.M.; Xavier, G.R.; Hax, F.; Rombaldi, C.V. Glyphosate and imazethapyr-induced effects on yield, nodule mass and biological nitrogen fixation in field-grown glyphosate-resistant soy-bean. Soil Biol. Biochem. 2009, 41, 420–422. [Google Scholar] [CrossRef] [Green Version]
- Bollich, P.K.; Dunigan, E.P.; Jadi, A.W.M. Effects of Seven Herbicides on N2 (C2 H2) Fixation by Soybeans. Weed Sci. 1985, 33, 427–430. [Google Scholar] [CrossRef]
- Soybean Nutrient Management Guidelines, University of Minnesota. Available online: https://drive.google.com/file/d/1VMhWf7uxmBu8WeIMTZsCEq1Yu7NMMZBY/view (accessed on 14 April 2022).
- Vlachostergios, D.; Noulas, C.; Baxevanos, D.; Raptopoulou, C.; Aggelopoulos, V.; Karanika, C.; Kantartzi, S.K.; Mavromatis, A. Response of early maturity soybean cultivars to row spacing in full-season crop and double-crop systems. Plant Soil Environ. 2021, 67, 18–25. [Google Scholar] [CrossRef]
- Specht, J.E.; Chase, K.; Macrander, M.; Graef, G.L.; Chung, J.; Markwell, J.P.; Germann, M.; Orf, J.H.; Lark, K.G. Soybean response to water: A QTL analysis of drought tolerance. Crop Sci. 2001, 41, 493–509. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Popović, V.; Tatić, M.; Spalević, V.; Rajičić, V.; Filipović, V.; Šarčević-Todosijević, L.J.; Stevanović, P. Effect of nitrogen fertilization on soybean plant height in arid year. In Proceedings of the 2nd International and 14th National Congress of Soil Science Society of Serbia “Solutions and Projections for Sustainable Soil Management”, Novi Sad, Srbia, 25–28 September 2017; pp. 65–73. [Google Scholar]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Souza, L.A.; Tavares, R. Nitrogen and Stem Development: A Puzzle Still to Be Solved. Front. Plant Sci. 2021, 12, 181. [Google Scholar] [CrossRef]
- Prahraj, C.S. Growth and Productivity of Soybean (Glycine max L. Merrill) as Affected by Interacting Influence of Rhizobium, Nitrogen and Potassium and Herbicide Use and Their Residual Effect on Wheat. Doctoral Thesis, Punjab Agricultural University, Ludhiana, India, 1994. [Google Scholar]
- Caliskan, S.; Ozkaya, I.; Caliskan, M.E.; Arslan, M. The effects of nitrogen and iron fertilization on growth, yield and fertilizer use efficiency of soybean in a Mediterranean-type soil. Field Crop. Res. 2008, 108, 126–132. [Google Scholar] [CrossRef]
- Kakabouki, I.; Folina, A.; Zisi, C.; Karydogianni, S. Fertilization expression via nitrogen indices in soybean crop under two system tillage. Not. Bot. Horti Agrobot. Cluj Napoca 2020, 48, 799–813. [Google Scholar] [CrossRef]
- Virk, H.K.; Singh, G.; Manes, G.S. Growth, symbiosis, productivity, and profitability of soybean at varying planting methods and nitrogen levels. J. Plant Nutr. 2018, 41, 1184–1196. [Google Scholar] [CrossRef]
- Tagliapietra, E.L.; Streck, N.A.; da Rocha, T.S.M.; Richter, G.L.; da Silva, M.R.; Cera, J.C.; Jerson Guedes, C.V.J.; Zanon, A.J. Optimum leaf area index to reach soybean yield potential in subtropical environment. Agron. J. 2018, 110, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Lawn, R.J.; Brun, W.A. Symbiotic Nitrogen Fixation in Soybeans. I. Effect of Photosynthetic Source-Sink Manipulations. Crop Sci. 1974, 14, 11–16. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Rogers, A.; Nelson, R.; Long, S.P. Testing the “source–sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric. For. Meteorol. 2004, 122, 85–94. [Google Scholar] [CrossRef]
- Basuchaudhuri, P. Source-sink relationships in soybean. Indian J. Plant Sci. 2016, 5, 19–25. [Google Scholar]
- Nagasuga, K. Soybean Seed Production and Canopy Photosynthesis in Cultivation. In Soybean-Biomass, Yield and Productivity; Kasai, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Voldeng, H.D.; Cober, E.R.; Hume, D.J.; Gillard, C.; Morrison, M.J. Fifty-eight years of genetic improvement of short-season soybean cultivars in Canada. Crop Sci. 1997, 37, 428–431. [Google Scholar] [CrossRef]
- Shibles, R.M.; Weber, C.R. Leaf area, solar radiation interception and dry matter production by soybeans. Crop Sci. 1965, 5, 575–577. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.; Shibles, R.M.; Byth, D.E. Effect of Plant Population and Row Spacing on Soybean Development and Production. Agron. J. 1966, 58, 99–102. [Google Scholar] [CrossRef]
- Hardman, L.L.; Brun, W.A. Effect of atmospheric carbon dioxide enrichment at different developmental stages on growth and yield components of soybeans. Crop Sci. 1971, 11, 886–888. [Google Scholar] [CrossRef]
- Board, J.E.; Harville, B.G. Soybean yield component responses to a light interception gradient during the reproductive period. Crop Sci. 1993, 33, 772–777. [Google Scholar] [CrossRef]
- Board, J.E.; Zhang, W.; Harville, B.G. Yield rankings for soybean cultivars grown in narrow and wide rows with late planting dates. Agron. J. 1996, 88, 240–245. [Google Scholar] [CrossRef]
- Gan, Y.; Stulen, I.; van Keulen, H.; Kuiper, P.J. Effect of N fertilizer top-dressing at various reproductive stages on growth, N2 fixation and yield of three soybean (Glycine max (L.) Merr.) genotypes. Field Crop. Res. 2003, 80, 147–155. [Google Scholar] [CrossRef]
- Dong, S.K.; Gong, Z.P.; Zu, W. Effects of nitrogen nutrition levels on N-accumulation and yields of soybean. Plant Nutr. Fertil. Sci. 2010, 16, 65–70. [Google Scholar]
- Mourtzinis, S.; Kaur, G.; Orlowski, J.M.; Shapiro, C.A.; Lee, C.D.; Wortmann, C.; Holshouser, D.; Nafziger, E.D.; Kandel, H.; Niekamp, J.; et al. Soybean response to nitrogen application across the United States: A synthesis-analysis. Field Crop. Res. 2018, 215, 74–82. [Google Scholar] [CrossRef]
- Capatana, N.; Bolohan, C.; Marin, D.I. Research regarding the influence of mineral fertilization along with Bradyrhizobium japonicum on soybean grain yield (Glycine max (L.) Merrill) under the conditions of south-east Romania. Sci. Pap. Ser. A Agron. 2017, 60, 207–214. [Google Scholar]
- Jadhav, A.S.; Andhale, R.P.; Patil, P.A. Effect of integrated nutrient management on yield attributes and yield of soybean. J. Maharashtra Agric. Univ. 2009, 34, 86–88. [Google Scholar]
- Lorenc-Kozik, A.M.; Pisulewska, E. Effect of increasing levels of nitrogen fertilizer and microelements on seed yield of selected soybean cultivars. Rośliny Oleiste-Oilseed Crop. 2003, 24, 131–142. [Google Scholar]
- Prusiński, J.; Baturo-Cieśniewska, A.; Borowska, M. Response of soybean (Glycine max (L.) Merrill) to mineral nitrogen fertilization and Bradyrhizobium japonicum seed inoculation. Agronomy 2020, 10, 1300. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I.; Findlay, W.I. Relationships among photosynthetic rate, bean yield and other characters in field-grown cultivars of soybean. Can. J. Plant Sci. 1981, 61, 190–197. [Google Scholar] [CrossRef]
- Saitoh, K.; Nishimura, K.; Kuroda, T. Characteristics of flowering and pod set in wild and cultivated types of soybean. Plant Prod. Sci. 2004, 7, 172–177. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Gulden, R.H.; Vessey, J.K. Low concentrations of ammonium inhibit specific nodulation (nodule number g−1 root DW) in soybean (Glycine max [L.] Merr.). Plant Soil 1998, 198, 127–136. [Google Scholar] [CrossRef]
- Cigelske, B.D.; Kandel, H.; DeSutter, T.M. Soybean Nodulation and Plant Response to Nitrogen and Sulfur Fertilization in the Northern US. Agric. Sci. 2020, 11, 592. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.M.; Abd-Alla, M.H. Effect of different rates of N-fertilizers on nodulation, nodule activities and growth of two field grown cvs. of soybean. Nutr. Cycl. Agroecosyst. 1995, 43, 37–41. [Google Scholar]
- Oklahoma Cooperative Extension Service. Available online: https://extension.okstate.edu/fact-sheets/print-publications/pss/understanding-soybean-nodulation-and-inoculation-pss-2169.pdf (accessed on 14 April 2022).
- Căpățână, N.; Bolohan, C.; Oprea, C.A.; Marin, D.I. Influence of Soil Tillage Systems and Inoculation on Soybean Nodulation and Yield. Sci. Pap.-Ser. A Agron. 2018, 61, 46–52. [Google Scholar]
- Bilalis, D.; Karkanis, A.; Pantelia, A.; Patsiali, S.; Konstantas, A.; Efthimiadou, A. Weed populations are affected by tillage systems and fertilization practices in organic flax (‘Linum usitatissimum’ L.) crop. Aust. J. Crop Sci. 2012, 6, 157–163. [Google Scholar]
- Tang, L.; Cheng, C.; Wan, K.; Li, R.; Wang, D.; Tao, Y.; Pan, J.; Xie, J.; Chen, F. Impact of fertilizing pattern on the biodiversity of a weed community and wheat growth. PLoS ONE 2014, 9, e84370. [Google Scholar] [CrossRef] [PubMed]
- Holzner, W.; Numata, M. Biology and Ecology of Weeds; Springer: Dordrecht, The Netherlands, 2013; p. 400. [Google Scholar]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii S. Watson and A. hybridus L. Can. J. Plant Sci. 2004, 84, 631–668. [Google Scholar]
- Edesi, L.; Jaervan, M.; Adamson, A.; Lauringson, E.; Kuht, J. Weed species diversity and community composition in conventional and organic farming: A five-year experiment. Zemdirb.-Agric. 2012, 99, 339–346. [Google Scholar]
- Chauhan, B.S.; Abugho, S.B. Effects of water regime, nitrogen fertilization, and rice plant density on growth and re-production of lowland weed Echinochloa crus-galli. Crop Prot. 2013, 54, 142–147. [Google Scholar] [CrossRef]
- Mekdad, A.A.; El-Enin, M.M.A.; Rady, M.M.; Hassan, F.A.; Ali, E.F.; Shaaban, A. Impact of Level of Nitrogen Fertilization and Critical Period for Weed Control in Peanut (Arachis hypogaea L.). Agronomy 2021, 11, 909. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Fried, G.; Petit, S.; Dessaint, F.; Reboud, X. Arable weed decline in Northern France: Crop edges as refugia for weed conservation? Biol. Conserv. 2009, 142, 238–243. [Google Scholar] [CrossRef]
- Schumacher, M.; Dieterich, M.; Gerhards, R. Effects of weed biodiversity on the ecosystem service of weed seed predation along a farming intensity gradient. Glob. Ecol. Conserv. 2020, 24, e01316. [Google Scholar] [CrossRef]
- Finger, R.; Buchmann, N. An ecological economic assessment of risk-reducing effects of species diversity in managed grasslands. Ecol. Econ. 2015, 110, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Schütte, G.; Eckerstorfer, M.; Rastelli, V.; Reichenbecher, W.; Restrepo-Vassalli, S.; Ruohonen-Lehto, M.; Saucy, W.A.G.; Mertens, M. Herbicide resistance and biodiversity: Agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ. Sci. Eur. 2017, 29, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyšek, P.; Lepš, J. Response of a weed community to nitrogen fertilization: A multivariate analysis. J. Veg. Sci. 1991, 2, 237–244. [Google Scholar] [CrossRef]
- Inouye, R.S.; Tilman, D. Convergence and divergence of old field vegetation after 11 years of nitrogen addition. Ecology 1995, 76, 1872–1887. [Google Scholar] [CrossRef]
- Chețan, F.; Chețan, C.; Bogdan, I.; Pop, A.I.; Moraru, P.I.; Rusu, T. The Effects of Management (Tillage, Fertilization, Plant Density) on Soybean Yield and Quality in a Three-Year Experiment under Transylvanian Plain Climate Conditions. Land 2021, 10, 200. [Google Scholar] [CrossRef]
- Lavres, J.; Castro Franco, G.; de Sousa Câmara, G.M. Soybean seed treatment with nickel improves biological nitrogen fixation and urease activity. Front. Environ. Sci. 2016, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Wang, H.; Ma, X.; Peng, H.; Shan, J. Modeling the current land suitability and future dynamics of global soybean cultivation under climate change scenarios. Field Crop. Res. 2021, 263, 108069. [Google Scholar] [CrossRef]
| Soil Type | Clay Loam |
|---|---|
| Clay | 29.3% |
| Silt | 34.9% |
| Sand | 35.8% |
| pH (1:1 H2O) | 7.38 |
| Organic matter | 3.11% |
| CaCO3 | 13.4% |
| Total Mineral Nitrogen | 0.156% |
| Phosphorus–P Olsen | 178 mg kg−1 soil |
| Potassium | 625 mg kg−1 soil |
| Index Name | Equation | References |
|---|---|---|
| [32] | ||
| [34] | ||
| [35] | ||
| [36] |
| LAI | Height (cm) | Plant Biomass (kg ha−1) | Yield (kg ha−1) | Number of Nodules per Plant | |
|---|---|---|---|---|---|
| Year A | |||||
| Control | 4.81 a | 42.83 a | 3863 a | 4095 a | 6.2 a |
| N80 | 5.09 b | 46.17 ab | 4021 ab | 4225 ab | 5.8 a |
| N100 | 5.21 bc | 49.33 b | 4141 bc | 4327 bc | 5.5 a |
| N120 | 5.62 c | 53.17 c | 4272 c | 4441 c | 5.3 a |
| Fertilization (F) | ** | *** | *** | ** | ns |
| Year B | |||||
| Control | 4.84 a | 44.33 a | 3754 a | 4066 a | 7.5 a |
| N80 | 5.02 ab | 48.50 b | 3962 b | 4208 ab | 7.0 a |
| N100 | 5.33 bc | 52.50 c | 4135 bc | 4343 bc | 6.5 a |
| N120 | 5.54 c | 54.33 c | 4258 c | 4462 c | 6.3 a |
| Fertilization (F) | *** | *** | ** | *** | ns |
| Overall effects | |||||
| Fertilization (F) | *** | *** | *** | *** | ns |
| Year (Y) | ns | ** | ns | ns | *** |
| F × Y | ns | ns | ns | ns | ns |
| Weed Density (m−2) | Total Weed Biomass (kg ha−1) | Number of Weed Species | |
|---|---|---|---|
| Year A | |||
| Control | 7.67 a | 411.7 a | 4.17 a |
| N80 | 9.50 b | 470.1 ab | 3.33 a |
| N100 | 11.01 bc | 528.3 b | 4.01 a |
| N120 | 14.03 c | 605.2 c | 4.50 a |
| Fertilization (F) | ** | *** | ns |
| Year B | |||
| Control | 9.67 a | 416.7 a | 4.33 a |
| N80 | 10.83 a | 478.3 a | 4.17 a |
| N100 | 13.04 ab | 531.7 b | 4.13 a |
| N120 | 14.33 b | 615.0 b | 4.50 a |
| Fertilization (F) | *** | *** | ns |
| Overall effects | |||
| Fertilization (F) | *** | *** | ns |
| Year (Y) | ns | ns | ns |
| (F) × (Y) | ns | ns | ns |
| Crop Resistance Index (CRI) | H (Shannon) Index | Dmg (Margalef) Index | J (Pielou) Index | |
|---|---|---|---|---|
| Year A | ||||
| Control | 1.00 a | 1.21 a | 3.78 a | 2.05 a |
| N80 | 0.91 a | 1.14 a | 4.24 a | 2.21 a |
| N100 | 0.84 ab | 1.16 a | 3.75 a | 1.98 a |
| N120 | 0.76 b | 1.30 a | 3.36 a | 2.01 a |
| Fertilization (F) | ** | ns | ns | ns |
| Year B | ||||
| Control | 1.00 a | 1.16 a | 3.72 a | 1.91 a |
| N80 | 0.91 a | 1.08 a | 3.70 a | 1.84 a |
| N100 | 0.86 ab | 1.13 a | 3.70 a | 1.89 a |
| N120 | 0.77 b | 1.15 a | 3.36 a | 1.78 a |
| Fertilization (F) | *** | ns | ns | ns |
| Overall effects | ||||
| Fertilization (F) | *** | ns | ns | ns |
| Year (Y) | ns | ns | ns | ns |
| (F) × (Y) | ns | ns | ns | ns |
| A. retroflexus | C. album | S. nigrum | E. crus-galli | |||||
|---|---|---|---|---|---|---|---|---|
| Year A | ||||||||
| Density | Biomass | Density | Biomass | Density | Biomass | Density | Biomass | |
| N0 | 5.33 ± 1.09 a | 70.50 ± 15.71 a | 3.50 ± 0.66 a | 56.73 ± 9.21 a | 1.33 ± 0.61 a | 26.57 ± 13.18 a | 1.67 ± 0.42 a | 14.47 ± 3.88 a |
| N80 | 6.00 ± 1.17 a | 103.84 ± 21.54 ab | 3.50 ± 0.43 a | 70.16 ± 15.52 a | 1.67 ± 0.67 a | 45.22 ± 17.18 a | 0.67 ± 0.33 a | 7.78 ± 0.55 a |
| N100 | 6.00 ± 1.22 a | 106.45 ± 21.86 ab | 3.33 ± 0.70 a | 81.86 ± 7.81 a | 1.50 ± 0.56 a | 45.03 ± 17.81 a | 0.83 ± 0.48 a | 9.47 ± 0.49 a |
| N120 | 6.83 ± 1.66 a | 138.47 ± 29.62 b | 2.83 ± 0.95 a | 83.69 ± 8.21 a | 2.83 ± 1.05 a | 60.42 ± 13.16 a | 1.67 ± 0.67 a | 17.25 ± 6.25 a |
| Year B | ||||||||
| Density | Biomass | Density | Biomass | Density | Biomass | Density | Biomass | |
| N0 | 5.83 ± 1.38 a | 83.14 ± 21.44 a | 2.67 ± 0.21 a | 38.41 ± 3.35 a | 1.67 ± 0.56 a | 36.92 ± 13.28 a | 1.50 ± 0.67 a | 15.75 ± 7.33 a |
| N80 | 5.83 ± 1.58 a | 106.20 ± 25.28 a | 2.83 ± 0.31 a | 48.48 ± 3.67 a | 2.33 ± 1.12 a | 53.13 ± 2.65 a | 1.17 ± 0.65 a | 12.67 ± 7.18 a |
| N100 | 8.17 ± 0.95 a | 157.23 ± 18.52 a | 2.00 ± 0.45 a | 51.90 ± 6.56 a | 1.33 ± 0.61 a | 36.92 ± 16.81 a | 2.00 ± 0.52 a | 22.58 ± 6.39 a |
| N120 | 10.33 ± 1.43 a | 175.04 ± 21.79 b | 2.17 ± 0.17 a | 54.89 ± 4.57 a | 1.17 ± 0.65 a | 47.32 ± 7.14 a | 1.67 ± 0.76 a | 17.38 ± 6.03 a |
| Yield | No. of Nodules | H Index | Dmg Index | J Index | CRI | Weed Density | Total Weed Biomass | |
|---|---|---|---|---|---|---|---|---|
| Soybean biomass | 0.1724 ns | −0.2962 ns | 0.3182 * | −0.1560 ns | 0.2661 ns | −0.5028 *** | 0.1847 ns | −0.2029 * |
| Yield | 0.3431 * | 0.1949 ns | 0.0120 ns | 0.1474 ns | −0.2624 ns | 0.1735 ns | 0.1548 ns | |
| No. of nodules | 0.1302 ns | 0.0394 ns | −0.1276 ns | 0.5030 *** | −0.0882 ns | −0.0556 ns | ||
| H index | −0.6225 *** | 0.9644 *** | −0.2911 * | 0.2926 * | 0.3315 * | |||
| Dmg index | −0.6780 *** | 0.0739 ns | 0.4854 *** | 0.3588 * | ||||
| J index | −0.2368 ns | 0.1420 ns | 0.2005 ns | |||||
| CRI | −0.2654 ns | −0.2546 * | ||||||
| Weed density | 0.7946 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakabouki, I.; Mavroeidis, A.; Kouneli, V.; Karydogianni, S.; Folina, A.; Triantafyllidis, V.; Efthimiadou, A.; Roussis, I.; Zotos, A.; Kosma, C.; et al. Effects of Nitrogen Fertilization on Weed Flora and Productivity of Soybean [Glycine max (L.) Merr.] Crop. Nitrogen 2022, 3, 284-297. https://doi.org/10.3390/nitrogen3020019
Kakabouki I, Mavroeidis A, Kouneli V, Karydogianni S, Folina A, Triantafyllidis V, Efthimiadou A, Roussis I, Zotos A, Kosma C, et al. Effects of Nitrogen Fertilization on Weed Flora and Productivity of Soybean [Glycine max (L.) Merr.] Crop. Nitrogen. 2022; 3(2):284-297. https://doi.org/10.3390/nitrogen3020019
Chicago/Turabian StyleKakabouki, Ioanna, Antonios Mavroeidis, Varvara Kouneli, Stella Karydogianni, Antigolena Folina, Vassilios Triantafyllidis, Aspasia Efthimiadou, Ioannis Roussis, Anastasios Zotos, Chariklia Kosma, and et al. 2022. "Effects of Nitrogen Fertilization on Weed Flora and Productivity of Soybean [Glycine max (L.) Merr.] Crop" Nitrogen 3, no. 2: 284-297. https://doi.org/10.3390/nitrogen3020019
APA StyleKakabouki, I., Mavroeidis, A., Kouneli, V., Karydogianni, S., Folina, A., Triantafyllidis, V., Efthimiadou, A., Roussis, I., Zotos, A., Kosma, C., & Katsenios, N. (2022). Effects of Nitrogen Fertilization on Weed Flora and Productivity of Soybean [Glycine max (L.) Merr.] Crop. Nitrogen, 3(2), 284-297. https://doi.org/10.3390/nitrogen3020019











