Secondary Immobilization as a Phase of N mineralization Dynamics of Soil Organic Inputs
Abstract
1. Introduction
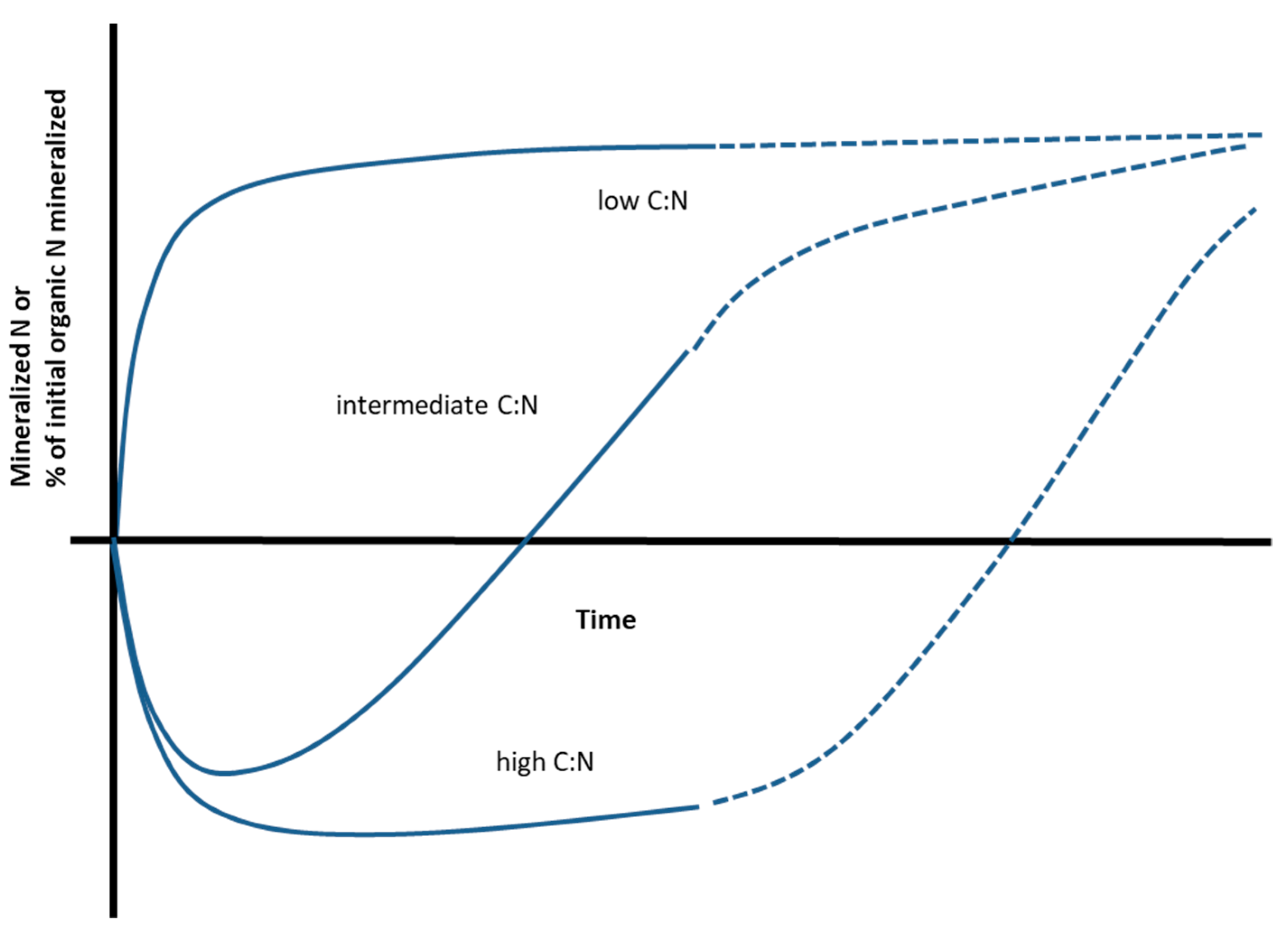
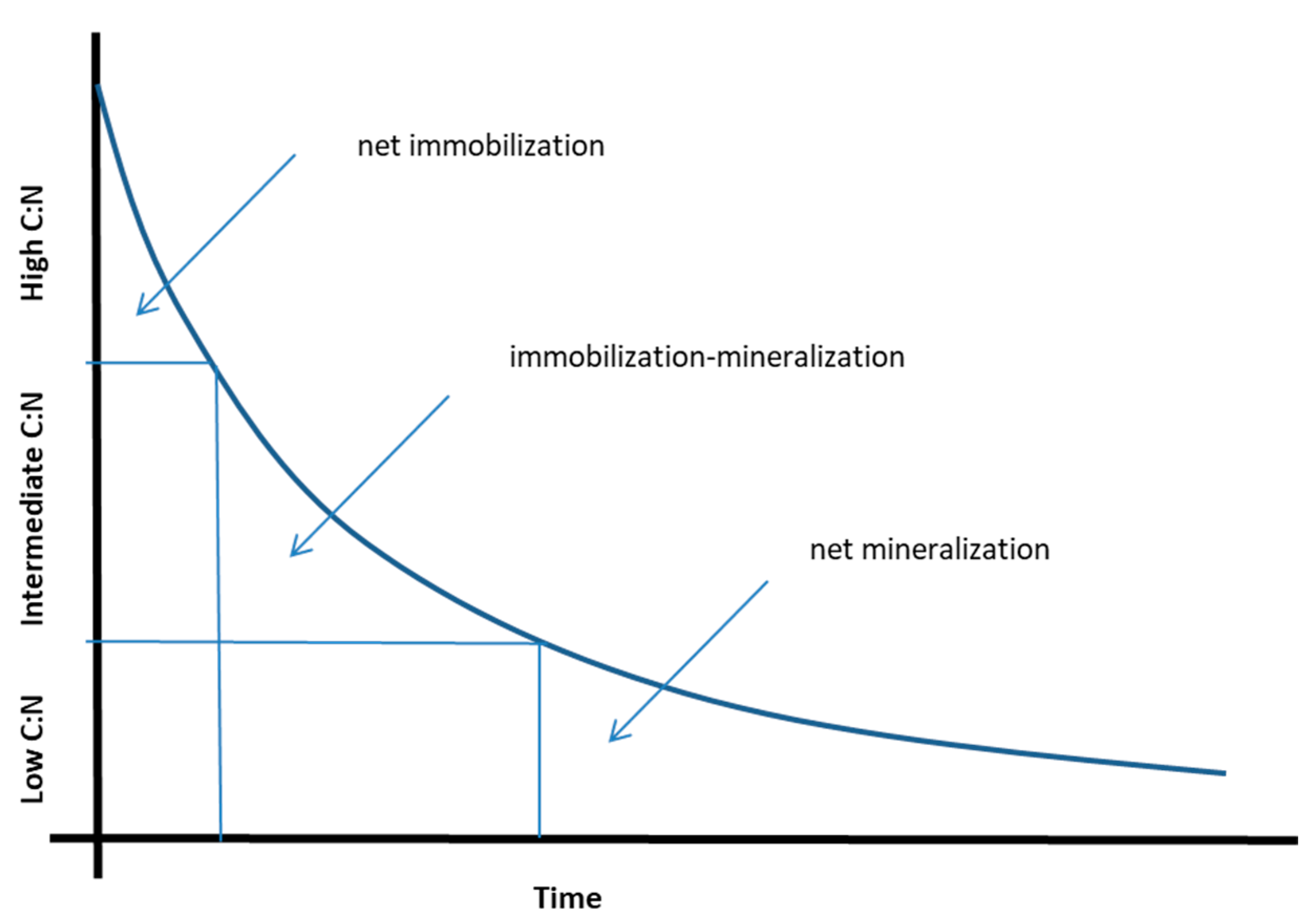
2. The Relationship between N mineralization and C:N Ratio for Processed Materials
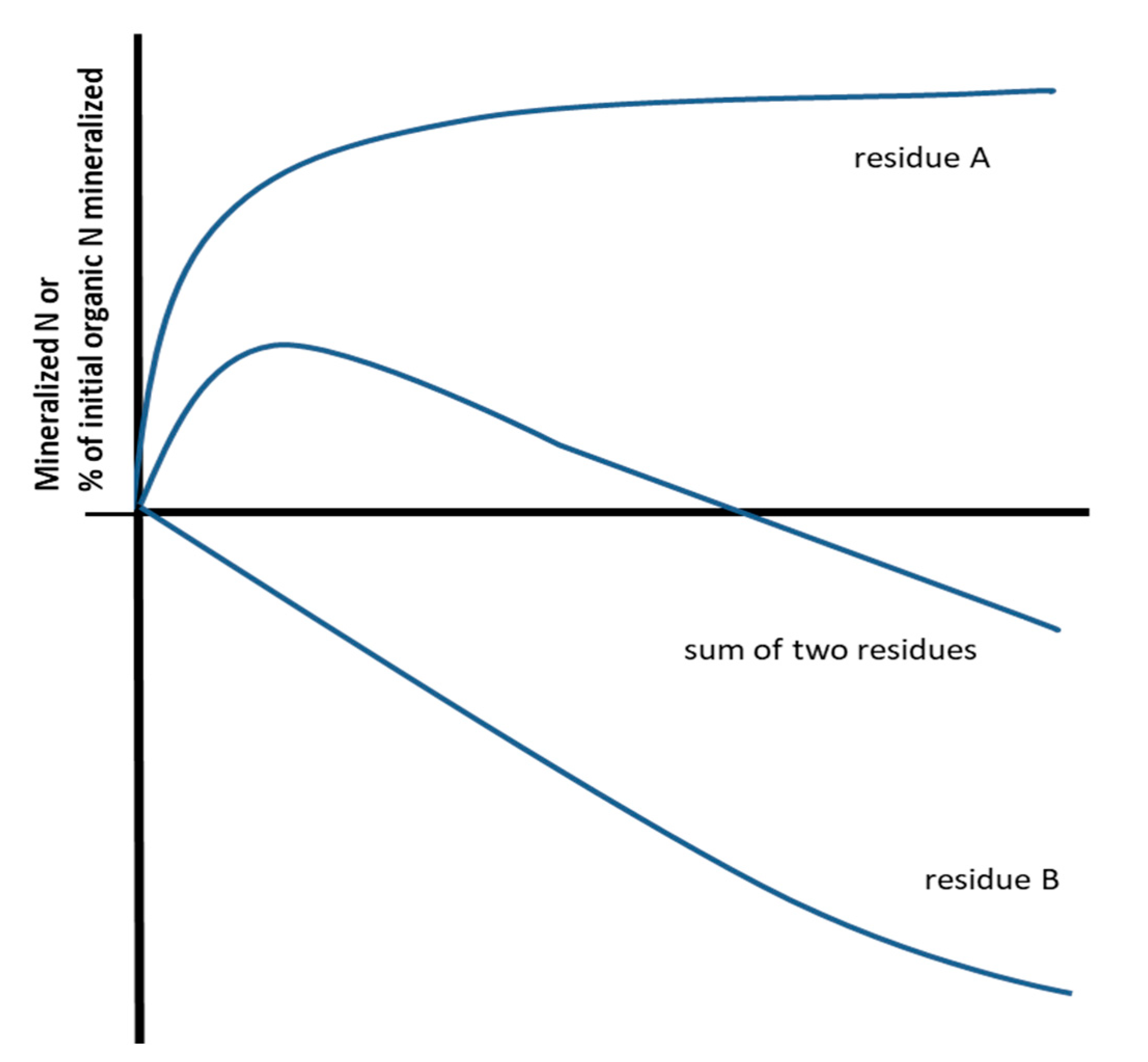
3. Secondary Immobilization
4. Potential Interpretations
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Stanford, G.; Smith, S.J. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Mary, B.; Rémy, J.C. Essai d’appréciation de la capacité de minéralisation de l’azote des sols de grande culture. Ann. Agron. 1979, 30, 513–527. [Google Scholar]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef]
- Pansu, M.; Thuriès, L.; Larré-Larrouy, M.C.; Bottner, P. Predicting N transformations from organic inputs in soil in relation to incubation time and biochemical composition. Soil Biol. Biochem. 2003, 35, 353–363. [Google Scholar] [CrossRef]
- Probert, M.E.; Delve, R.J.; Kimani, S.K.; Dimes, J.P. Modelling nitrogen mineralization from manures: Representing quality aspects by varying C:N ratio of sub-pools. Soil Biol. Biochem. 2005, 37, 279–287. [Google Scholar] [CrossRef]
- Levavasseur, F.; Lashermes, G.; Mary, B.; Morvan, T.; Nicolardot, B.; Parnaudeau, V.; Thuriès, L.; Houot, S. Quantifying and simulating carbon and nitrogen mineralization from diverse exogenous organic matters. Soil Use Manag. 2021, 38, 411–425. [Google Scholar] [CrossRef]
- Anderson, J.M. The breakdown and decomposition of sweet chestnut (Castanea sativa Mill.) and beech (Fagus sylvatica L.) leaf litter in two deciduous woodland soils. Oecologia 1973, 12, 251–274. [Google Scholar] [CrossRef]
- Dalias, P.; Anderson, J.M.; Bottner, P.; Coûteaux, M.M. Temperature responses of carbon mineralization in conifer forest soils from different regional climates incubated under standard laboratory conditions. Glob. Chang. Biol. 2001, 7, 181–192. [Google Scholar] [CrossRef]
- Dalias, P.; Anderson, J.M.; Bottner, P.; Coûteaux, M.M. Temperature responses of net nitrogen mineralization and nitrification in conifer forest soils incubated under standard laboratory conditions. Soil Biol. Biochem. 2002, 34, 691–701. [Google Scholar] [CrossRef]
- Calderon, F.J.; McCarty, G.W.; Reeves, J.B. Analysis of manure and soil nitrogen mineralization during incubation. Biol. Fertil. Soils 2005, 41, 328–336. [Google Scholar] [CrossRef]
- Paul, J.W.; Beauchamp, E.G. Short-term nitrogen dynamics in soil amended with fresh and composted cattle manures. Can. J. Soil Sci. 1994, 74, 147–155. [Google Scholar] [CrossRef]
- Griffin, T.S.; Hutchinson, M. Compost maturity effects on nitrogen and carbon mineralization and plant growth. Compost Sci. Util. 2007, 15, 228–236. [Google Scholar] [CrossRef]
- Ebid, A.; Ueno, H.; Ghoneim, A. Nitrogen mineralization kinetics and nutrient availability in soil amended with composted tea leaves, coffee waste, and kitchen garbage. Int. J. Soil Sci. 2007, 2, 96–106. [Google Scholar]
- De Neve, S.; Hofman, G. Modelling N mineralization of vegetable crop residues during laboratory incubations. Soil Biol. Biochem. 1996, 28, 1451–1457. [Google Scholar] [CrossRef]
- Vityakon, P.; Dangthaisong, N. Environmental influences on nitrogen transformation of different quality tree litter under submerged and aerobic conditions. Agrofor. Syst. 2005, 63, 225–236. [Google Scholar] [CrossRef]
- Moreno-Cornejo, J.; Zornoza, R.; Faz, A. Carbon and nitrogen mineralization during decomposition of crop residues in a calcareous soil. Geoderma 2014, 230, 58–63. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Tahir Mahmood, M.; Sabir, N.; Khurshid, M. Impact of the addition of different plant residues on nitrogen mineralization–immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth 2015, 6, 197–205. [Google Scholar] [CrossRef]
- Li, H.C.; Hu, Y.L.; Mao, R.; Zhao, Q.; Zeng, D.H. Effects of nitrogen addition on litter decomposition and CO2 release: Considering changes in litter quantity. PLoS ONE 2015, 10, e0144665. [Google Scholar] [CrossRef]
- Ansong Omari, R.; Bellingrath-Kimura, D.S.; Fujii, Y.; Sarkodee-Addo, E.; Appiah Sarpong, K.; Oikawa, Y. Nitrogen mineralization and microbial biomass dynamics in different tropical soils amended with contrasting organic resources. Soil Syst. 2018, 2, 63. [Google Scholar] [CrossRef]
- Zaouchi, Y. Dynamique de la minéralisation du carbone et de l’azote organiques dans des sols d’origines pédoclimatiques différentes: Une expérience d’incubation. J. New Sci. 2015, 19, 748–758. [Google Scholar]
- Azeez, J.O.; Van Averbeke, W. Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Bioresour. Technol. 2010, 101, 5645–5651. [Google Scholar] [CrossRef] [PubMed]
- Keskinen, R.; Saastamoinen, M.; Nikama, J.; Särkijärvi, S.; Myllymäki, M.; Salo, T.; Uusi-Kämppä, J. Recycling nutrients from horse manure: Effects of bedding type and its compostability. Agric. Food Sci. 2017, 26, 68–79. [Google Scholar] [CrossRef]
- Olowoboko, T.B.; Azeez, J.O.; Olujimi, O.O.; Babalola, O.A. Availability and dynamics of organic carbon and nitrogen indices in some soils amended with animal manures and ashes. Int. J. Recycl. Org. Waste Agric. 2018, 7, 287–304. [Google Scholar] [CrossRef]
- Szogi, A.A.; Shumaker, P.D.; Ro, K.S.; Sigua, G.C. Nitrogen mineralization in a sandy soil amended with treated low-phosphorus broiler litter. Environments 2019, 6, 96. [Google Scholar] [CrossRef]
- Dalias, P.; Christou, A. Nitrogen supplying capacity of animal manures to the soil in relation to the length of their storage. Nitrogen 2020, 1, 52–66. [Google Scholar] [CrossRef]
- Daramy, M.A.; Kawada, R.; Oba, S. Alterations of the chemical compositions, surface functionalities, and nitrogen structures of cage layer chicken manure by carbonization to improve nitrogen bioavailability in soil. Agronomy 2020, 10, 1031. [Google Scholar] [CrossRef]
- Islam, M.R.; Bilkis, S.; Hoque, T.S.; Uddin, S.; Jahiruddin, M.; Rahman, M.M.; Rahman, M.M.; Alhomrani, M.; Hossain, M.A. Mineralization of farm manures and slurries for successive release of carbon and nitrogen in incubated soils varying in moisture status under controlled laboratory conditions. Agriculture 2021, 11, 846. [Google Scholar] [CrossRef]
- Killham, K. Soil Ecology; Cambridge University Press: Cambridge, UK, 1994; p. 242. [Google Scholar]
- Wang, R.; Feng, Q.; Liao, T.; Zheng, X.; Butterbach-Bahl, K.; Zhang, W.; Jin, C. Effects of nitrate concentration on the denitrification potential of a calcic cambisol and its fractions of N2, N2O and NO. Plant Soil 2013, 363, 175–189. [Google Scholar] [CrossRef]
- Guggenberger, G. Humification and mineralization in soils. In Microorganisms in Soils: Roles in Genesis and Functions; Buscot, F., Varma, A., Eds.; Springer: Berlin, Germany, 2005; pp. 85–106. [Google Scholar]
- Kopittke, P.M.; Dalal, R.C.; Hoeschen, C.; Li, C.; Menzies, N.W.; Mueller, C.W. Soil organic matter is stabilized by organo-mineral associations through two key processes: The role of the carbon to nitrogen ratio. Geoderma 2020, 357, 113974. [Google Scholar] [CrossRef]
- Hedges, J.I.; Eglinton, G.; Hatcher, P.G.; Kirchman, D.L.; Arnosti, C.; Derenne, S.; Evershed, R.P.; Kögel-Knabner, I.; de Leeuw, J.W.; Littke, R.; et al. The molecularly uncharacterized component of nonliving organic matter in natural environment. Org. Geochem. 2000, 31, 945–958. [Google Scholar] [CrossRef]
- Melillo, J.M.; Naiman, R.J.; Aber, J.D.; Linkins, A.E. Factors controlling mass loss and nitrogen dynamics of plant litter decaying in northern streams. Bull. Mar. Sci. 1984, 35, 341–356. [Google Scholar]
- Berg, B. Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manag. 2000, 133, 13–22. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalias, P.; Christou, A. Secondary Immobilization as a Phase of N mineralization Dynamics of Soil Organic Inputs. Nitrogen 2022, 3, 600-607. https://doi.org/10.3390/nitrogen3040039
Dalias P, Christou A. Secondary Immobilization as a Phase of N mineralization Dynamics of Soil Organic Inputs. Nitrogen. 2022; 3(4):600-607. https://doi.org/10.3390/nitrogen3040039
Chicago/Turabian StyleDalias, Panagiotis, and Anastasis Christou. 2022. "Secondary Immobilization as a Phase of N mineralization Dynamics of Soil Organic Inputs" Nitrogen 3, no. 4: 600-607. https://doi.org/10.3390/nitrogen3040039
APA StyleDalias, P., & Christou, A. (2022). Secondary Immobilization as a Phase of N mineralization Dynamics of Soil Organic Inputs. Nitrogen, 3(4), 600-607. https://doi.org/10.3390/nitrogen3040039






