Abstract
In higher plants, hydrogen sulfide (H2S) is a recognized signaling molecule that performs multiple regulatory functions. The enzyme L-cysteine desulfhydrase (LCD) catalyzes the conversion of L-cysteine (L-Cys) to pyruvate and ammonium with the concomitant generation of H₂S, and it is considered one of the main sources of H2S in plants. Using non-denaturing polyacrylamide gel electrophoresis (PAGE) in combination with a specific assay for LCD activity, this study aims to identify the potential LCD isozymes in wild-type Arabidopsis thaliana seedlings of 16 days old grown under in vitro conditions, and to evaluate the potential impact of nitric oxide (NO) and H2S on these LCD isozymes. For this purpose, an Atnoa1 mutant characterized to have a low endogenous NO content as well as the exogenous application of H2S were used. Five LCD isozymes were detected, with LCD IV being the isozyme that has the highest activity. However, the LCD V activity was the only one that was positively modulated in the Atnoa1 mutants and by exogenous H2S. To our knowledge, this is the first report showing the different LCD isozymes present in Arabidopsis seedlings and how their activity is affected by NO and H2S content.
1. Introduction
Hydrogen sulfide (H2S) is a molecule that is endogenously generated in plant cells and, in recent years, it has gained great relevance due to its signaling function under physiological processes such as photosynthesis, seed germination, root growth, fruit ripening, and organ senescence, but also in the mechanism of response against stressful conditions [1,2,3,4]. Recently, in the model plant Arabidopsis thaliana, an in silico analysis was performed to find out how the H2S is generated as part of the sulfate assimilation and in the cysteine (Cys) metabolism [5]. Among the twenty-six identified enzymes involved in the metabolism of H2S, five enzymes directly generate H2S in plants including the cytosolic L-cysteine desulfhydrase (LCD), bifunctional cystathionine γ-lyase/cysteine synthase (DES1), the chloroplastic sulfite reductase (SiR), the mitochondrial bifunctional D-cysteine desulfhydrase/1-aminocyclopropane-1-carboxylate deaminase (DCDES1), and D-cysteine desulfhydrase 2 (DCDES2) [5,6,7]. The cytosolic LCD is considered one of the primary sources of H2S, which catalyzes the following reaction: L-cysteine + H2O → pyruvate + NH4+ + H2S + H+, using pyridoxal 5-phosphate (PLP) as a necessary cofactor [8,9,10,11].
Although the enzymatic source of nitric oxide (NO) in plants is still under debate [12], what is well established is that NO and derived species designated as reactive nitrogen species (RNS) constitute a family of molecules that exert a wide spectrum of functions in the physiology of plants and the mechanisms of responses to environmental stresses [13,14,15,16,17,18,19,20,21].
The participation of both molecules in plant cells is a research area of great interest, and an increased number of studies indicate that they can act both synergistically and antagonistically in different physiological processes [22,23] and in response to stress [2,24,25,26]. Furthermore, it has been seen that both NO and H2S can regulate protein function through post-translational modifications such as nitration, S-nitrosation, and persulfidation. Thus, some enzymes such as catalase, lipoxygenase, NADP-dependent dehydrogenase, NADP malic enzyme, or peroxidase undergo modifications via both molecules affecting their functions either positively or negatively [27,28,29,30,31].
Based on previous work, where an optimized method in polyacrylamide gels under non-denaturing conditions allowed the identification of different LCD isoenzymes in several species of the genus Allium and pepper fruit [8,11], the present study aims to analyze the isozymatic pattern of LCD in Arabidopsis seedlings and evaluate if any of these LCD isozymes could be modulated via NO and/or H2S.
2. Materials and Methods
2.1. Arabidopsis Material and Growth Conditions
The Arabidopsis thaliana ecotype Columbia wild type (Wt) and the Atnoa1 (Arabidopsis NO-associated 1) mutant, which has a deficit in the production of endogenous nitric oxide (NO), were used in this study. Seeds were surface sterilized as previously described [32]. Briefly, seeds were incubated for 5 min in 70% (v/v) ethanol solution containing 0.1% (w/v) SDS, then placed for 20 min in sterile water containing 20% (v/v) bleach and 0.1% (w/v) SDS, and washed four times in sterile water. The seeds were sown for 2 days at 4 °C in the dark for vernalization. Then, the seeds were placed on Petri dishes 90 mm in diameter containing a growth medium composed of 4.32 g/L commercial Murashige and Skoog (MS) medium (Sigma-Aldrich, Madrid, Spain) with a pH of 5.5, containing 1% (w/v) sucrose and 0.8% (w/v) phyto-agar. For the experiments with sodium hydrosulfide (NaHS) as H2S donor, a solution of 200 µM NaHS (final concentration) in sterile water was added to the Petri dishes [33]. All procedures were carried out in a laminar flow cabinet. Then, seedlings were grown for 16 days under long-day conditions (16 h light at 22 °C and 8 h dark at 18 °C), and a light intensity of 100 μE m−2·s−1.
2.2. Morphological Analyses of Arabidopsis thaliana Root System
Fresh weight and primary root length were evaluated in sixty A. thaliana Wt and Atnoa1 seedlings per treatment and expressed in mg per seedling and mm (mean ± SE), respectively. The Petri dishes containing the seedlings were scanned at 1200 dpi with Epson Perfection 2450 using Vuescan 9.0.94 software and the primary root length was analyzed with RootNav image analysis tool (University of Nottingham, Ver. 1.8.1, 64-bit).
2.3. Crude Extracts of Plant Tissues and Protein Assay
Arabidopsis seedlings of 16 days were collected and frozen in liquid nitrogen. Then, they were ground in a mortar with a pestle. The powder was suspended in a homogenizing medium (ratio 1:3, w/v) containing 100 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.1 (v/v) Triton X-100 and 10% (v/v) glycerol, and homogenates were centrifuged at 27,000× g for 20 min at 4 °C. The supernatants were used for the assays.
Protein content in the samples was determined using the Bio-Rad Protein Assay (Hercules, CA, USA), with bovine serum albumin as standard.
2.4. In-Gel Profile of L-Cysteine Desulfhydrase (LCD) Activity
Arabidopsis samples were separated non-denaturing polyacrylamide gel electrophoresis (PAGE) on 8% acrylamide gels. After the electrophoresis, to visualize the LCD (EC 4.4.1.28) activity bands, the gels were incubated in the dark in a staining buffer containing Tris-HCl 100 mM, pH 7.5, L-cysteine 20 mM, lead acetate 0.4 mM, pyridoxal 50-phosphate hydrate 50 µM and dithiothreitol 5 mM, until the appearance of brown bands over a colorless background [10,11]. Two internal controls were accomplished to check the specificity of the LCD activity; one was to incubate the gel in the absence of the substrate L-cysteine or the cofactor pyridoxal 5′-phosphate. In both cases, no band activity was detected.
2.5. Statistical Analysis
It was carried out using SPP program in which a one-way ANOVA was applied accompanied by Tukey’s post hoc test with a p level of 0.05.
3. Results and Discussion
The involvement of H2S in numerous physiological plant processes as well as in response to diverse types of environmental stresses is a subject of an increasing number of studies [2,3]. In plant cells, there are different enzymatic sources of H2S, and cytosolic L-cysteine desulfhydrase (LCD) is one of them [5]. Using Arabidopsis seedlings, this work has the goal to identify the presence of LCD isoenzymes and if they could be modulated by NO or H2S.
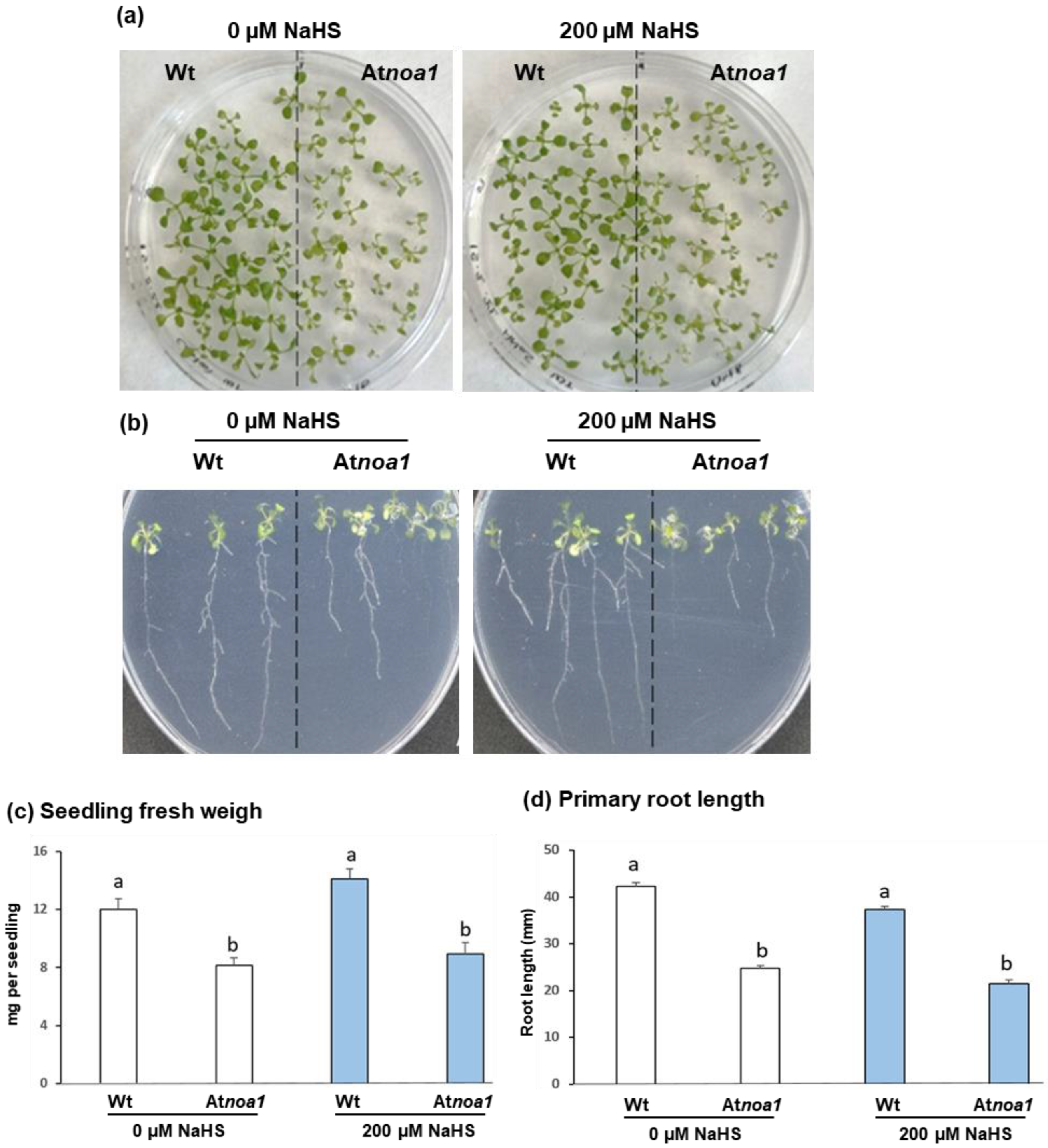
Figure 1 shows the appearance of Arabidopsis Wt and Atnoa1 seedlings grown in the absence and the presence of 200 µM NaHS (Figure 1a,b), as well as the analysis of their biomass (Figure 1c) and primary root length (Figure 1d) after 16 days. A visual examination allows observing that the green cotyledons of the Atnoa1 seedlings had smaller size and a yellowish color in comparison to Wt seedlings. This aspect should be associated with the NO deficiency in this mutant, which is likely related to cotyledon organogenesis and chloroplast formation. A similar situation occurred in previous assays with these mutants and the cyclic GTPase family, where smaller yellowish and pale green leaves were observed [34,35]. On the other hand, through comparing the color of the leaves from the mutant seedlings treated with H2S (Atnoa1 plus NaHS) with the control mutant seedlings, it is observed that the treated plantlets are greener than the untreated ones. Therefore, it is suggested that the treated seedlings have a lower loss of green color, similar to what occurs in pak choi plants (Brassica rapa subsp. chinensis) fumigated with H2S [36].
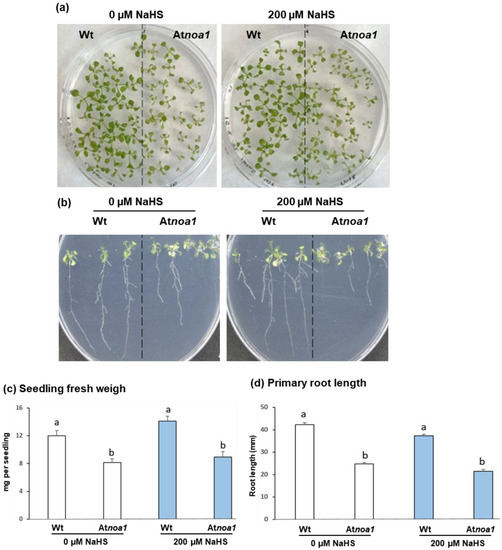
Figure 1.
Effect of the H2S donor NaHS on Arabidopsis seedling growth. (a) Appearance of 16-day-old Arabidopsis Wt and Atnoa1 seedlings grown in a horizontal position in MS medium supplemented and non-supplemented with 200 µM NaHS. (b) Appearance of 16-day-old Arabidopsis Wt and Atnoa1 seedlings grown in a vertical position in the same conditions of panel (a). (c) Arabidopsis seedling fresh weight (mg per seedling). (d) Primary root length per plant. Different letters indicate significant differences (p < 0.05).
When seedlings were grown in Petri dishes placed in a vertical position (Figure 1b,d), it was possible to study if there were changes in the root phenotype between Wt and Atnoa1 seedlings untreated and treated with H2S. The analysis of the length of the primary root of untreated seedlings allowed to observe that Wt seedlings were 42% higher than that of Atnoa1 mutants. Finally, in H2S-treated plants, the primary root length in Wt seedlings was 51% higher than that determined in the Atnoa1 mutants.
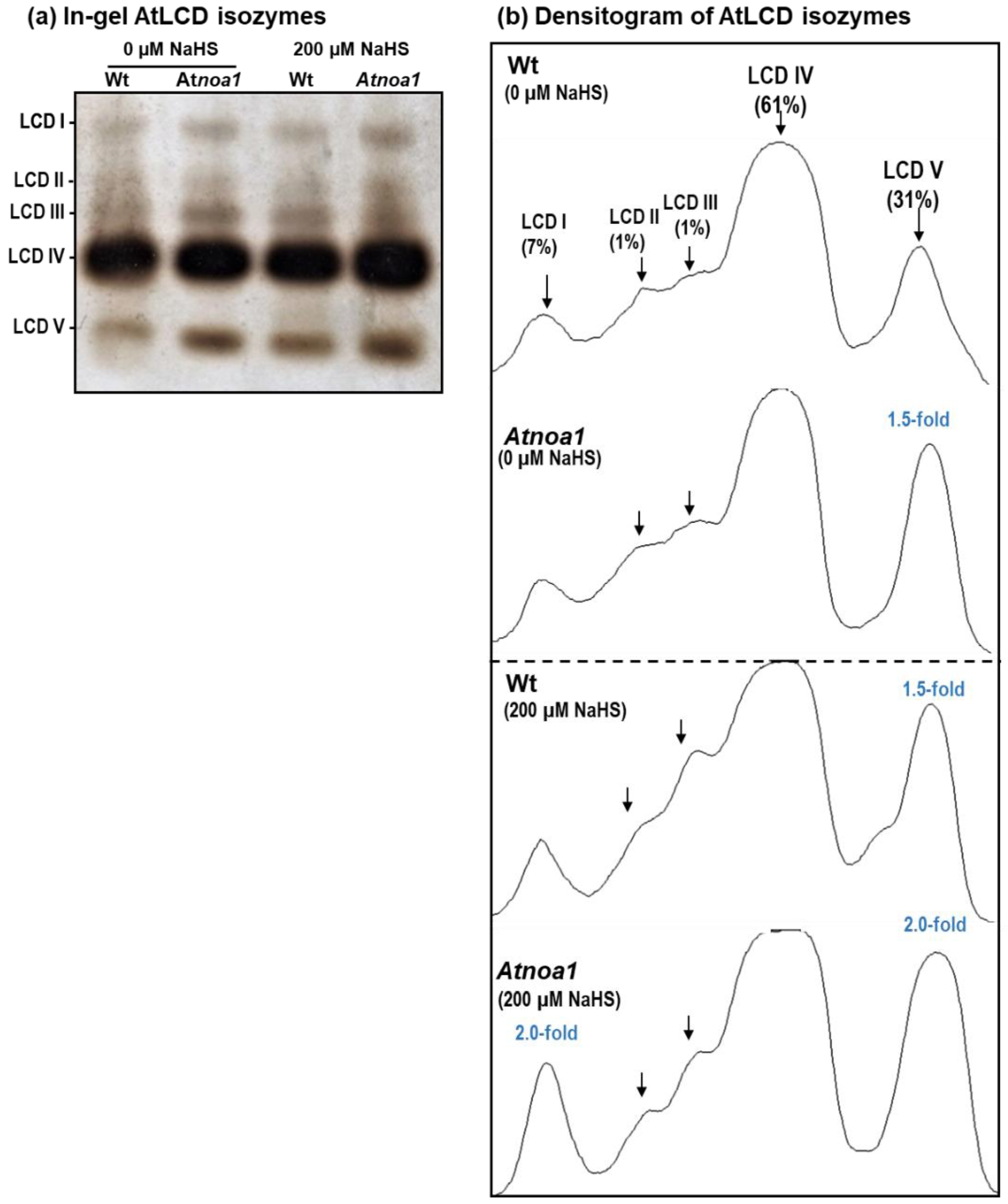
With the aim to identify the presence of different LCD isozymes, in-gel analyses were performed using 16-day-old Arabidopsis seedlings. Figure 2a displays the identification of five LCD isozymes designated AtLCD I to AtLCD V, according to their increasing electrophoretic mobility in the gel. AtLCD IV was the most prominent isozyme whereas AtLCD II was the least abundant. As shown in the figure, both NO and H2S influenced the zymogram, with AtLCD V being the most affected isozyme.
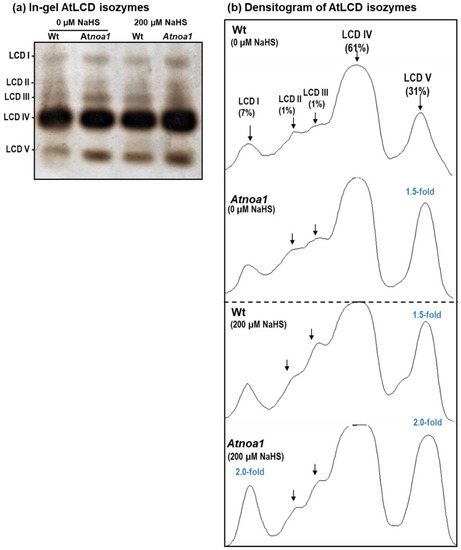
Figure 2.
(a) In-gel isozyme profile of L-cysteine desulfhydrase (LCD) activity in 16-day-old Arabidopsis thaliana Wt and Atnoa1 seedlings grown in MS medium supplemented and not supplemented with 200 µM NaHS. Protein samples (40 µg protein per lane) were separated using non-denaturing polyacrylamide gel electrophoresis on 8% acrylamide gels. LCD activity was detected using lead acetate staining (see Section 2 for details). (b) Densitometric analysis of LCD isozymes and their relative quantification (%) was made with the ImageJ 1.45 program. The numbers in parentheses indicate the fold increase in activity compared to the corresponding isozyme detected in Wt no supplemented with 200 µM NaHS.
Figure 2b depicts the total relative activity quantification considering all the LCD isozymes present in the Wt seedling (control). Thus, AtLCD IV represented 61% of the total activity, followed by AtLCD V with 31%, and AtLCD I with 7%, whereas AtLCD II and AtLCD III represented less than 1% each. Among the different isozymes, AtLCD V is the isozyme that underwent the greatest change in comparison to the Wt seedlings not supplemented with NaHS (control). Thus, it is observed that the Atnoa1 mutant increases 1.5-fold, and in seedlings supplemented with 200 µM NaHS, the increase in Wt is also 1.5-fold while in Atnoa1 it is 2-fold. On the other hand, the AtLCD I in Atnoa1 seedlings grown in a medium with 200 NaHS increases 3-fold in comparison to the control Wt seedlings grown without NaHS and 2-fold to the Wt seedlings supplemented with 200 µM NaHS.
There is currently growing evidence of the intimate interaction between H2S and NO in different physiological processes such as root development, stomatal movement, and plant cell death [37,38] as well as in response to environmental stress. For example, in maize (Zea mays L.) seedlings exposed to chromium stress, the exogenous application of NO and H2S protect plasma membrane integrity through upregulating H+-ATPase and glyoxalase pathways and through keeping optimal GSH content [39]. Furthermore, they also alleviate chromium toxicity through stimulating the antioxidant system and interfering with the Cr accumulation in roots, since it seems that these gasotransmitters stimulate the biosynthesis of phytochelatins [40]. In many cases, these molecules act via modulating the metabolism of reactive oxygen species (ROS) both in antioxidant systems and in ROS-generating enzymes [2,27,41,42]. Regulation is usually performed through posttranslational modifications (PTMs) such as nitration, S-nitrosation, and persulfidation [28,29,30,31,43]. The best-known examples are the antioxidant enzymes that scavenge H2O2, such as catalase and ascorbate peroxidase (APX). While catalase is inhibited via nitration, S-nitrosation, and persulfidation [7,27], APX is positively modulated via S-nitrosation and persulfidation but inhibited via nitration [44,45,46]. Recently, it has been shown that DES1 undergoes persulfidation, which regulates its activity [42], and also, the pepper cytosolic LCD is downregulated via nitration [10]. Taken together, all these data support a complex network between the metabolism of NO, H2S, and ROS, which might drive plant physiology under diverse conditions.
4. Conclusions
The enzyme L-cysteine desulfhydrase is recognized as one of the main sources of H2S in the cytosol of plant cells. To our knowledge, there are very few works that have analyzed the existence of different LCD isoenzymes in plants [8,10], so the existence of up to five LCD isoenzymes in Arabidopsis seedlings provides a piece of knowledge about this enzymatic system which can be very useful for future work. Furthermore, among the identified enzymes, LCD V seems to be positively modulated by low NO levels as well as by H2S itself, demonstrating the close relationship between both molecules in the sulfur metabolic pathway. Very recently, it has been reported that the LCD activity of pepper fruits undergoes a double regulation by NO, since S-nitrosation has a positive effect on the activity whereas the nitration triggered its inhibition [10].
Author Contributions
J.D.L.O.-S. and M.A.M.-V. performed experiments. F.J.C. and J.M.P. designed the work and drove and coordinated the tasks. F.J.C. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Our research is supported by a European Regional Development Fund co-financed grants from the Ministry of Science and Innovation (PID2019-103924GB-I00), the AEI (10.13039/501100011033) and Junta de Andalucía (P18-274 FR-1359), Spain.
Acknowledgments
The valuable technical assistance of Carmelo Ruiz-Torres is deeply acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corpas, F.J. Hydrogen Sulfide: A New Warrior against Abiotic Stress. Trends Plant Sci. 2019, 24, 983–988. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Corpas, F.J. Nitric Oxide and Hydrogen Sulfide Coordinately Reduce Glucose Sensitivity and Decrease Oxidative Stress via Ascorbate-Glutathione Cycle in Heat-Stressed Wheat (Triticum aestivum L.) Plants. Antioxidants 2021, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Tabassum, J.; Mubarik, M.S.; Anwar, S.; Zahra, N.; Sharif, Y.; Hafeez, M.B.; Zhang, C.; Corpas, F.J.; Chen, H. Hydrogen sulfide: An emerging component against abiotic stress in plants. Plant Biol. 2022, 24, 540–558. [Google Scholar] [CrossRef]
- González-Gordo, S.; Palma, J.M.; Corpas, F.J. Appraisal of H2S metabolism in Arabidopsis thaliana: In silico analysis at the subcellular level. Plant Physiol. Biochem. 2020, 155, 579–588. [Google Scholar] [CrossRef]
- Alvarez, C.; Calo, L.; Romero, L.C.; García, I.; Gotor, C. An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol. 2010, 152, 656–669. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. H2S in Horticultural Plants: Endogenous Detection by an Electrochemical Sensor, Emission by a Gas Detector, and Its Correlation with L-Cysteine Desulfhydrase (LCD) Activity. Int. J. Mol. Sci. 2022, 23, 5648. [Google Scholar] [CrossRef]
- Kurmanbayeva, A.; Bekturova, A.; Soltabayeva, A.; Oshanova, D.; Nurbekova, Z.; Srivastava, S.; Tiwari, P.; Dubey, A.K.; Sagi, M. Active O-acetylserine-(thiol) lyase A and B confer improved selenium resistance and degrade L-Cys and L-SeCys in Arabidopsis. J. Exp. Bot. 2022, 73, 2525–2539. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; López-Jaramillo, J.; González-Gordo, S.; Paradela, A.; Palma, J.M.; Corpas, F.J. H2S-generating cytosolic L-cysteine desulfhydrase (LCD) and mitochondrial D-cysteine desulfhydrase (DCD) from sweet pepper (Capsicum annuum L.) are regulated during fruit ripening and by nitric oxide (NO). Antioxid. Redox Signal. 2023. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; Rodríguez-Ruiz, M.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Analysis of Plant L-Cysteine Desulfhydrase (LCD) Isozymes by Non-denaturing Polyacrylamide Gel Electrophoresis. Methods Mol. Biol. 2023, 2642, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. NO source in higher plants: Present and future of an unresolved question. Trends Plant Sci. 2022, 27, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B.; Carreras, A.; Valderrama, R.; Palma, J.M.; León, A.M.; Sandalio, L.M.; del Río, L.A. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 2006, 224, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Chaki, M.; Fernández-Ocaña, A.; Valderrama, R.; Palma, J.M.; Carreras, A.; Begara-Morales, J.C.; Airaki, M.; Del Río, L.A.; Barroso, J.B. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008, 49, 1711–1722. [Google Scholar] [CrossRef]
- Signorelli, S.; Corpas, F.J.; Borsani, O.; Barroso, J.B.; Monza, J. Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 2013, 201–202, 137–146. [Google Scholar] [CrossRef]
- Manai, J.; Gouia, H.; Corpas, F.J. Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J. Plant Physiol. 2014, 171, 1028–1035. [Google Scholar] [CrossRef]
- Kolbert, Z.; Barroso, J.; Brouquisse, R.; Corpas, F.; Gupta, K.; Lindermayr, C.; Loake, G.; Palma, J.; Petřivalský, M.; Wendehenne, D.; et al. A forty year journey: The generation and roles of NO in plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef]
- Bhat, J.A.; Ahmad, P.; Corpas, F.J. Main nitric oxide (NO) hallmarks to relieve arsenic stress in higher plants. J. Hazard. Mater. 2021, 406, 124289. [Google Scholar] [CrossRef]
- Zuccarelli, R.; Rodríguez-Ruiz, M.; Lopes-Oliveira, P.J.; Pascoal, G.B.; Andrade, S.C.S.; Furlan, C.M.; Purgatto, E.; Palma, J.M.; Corpas, F.J.; Rossi, M.; et al. Multifaceted roles of nitric oxide in tomato fruit ripening: NO-induced metabolic rewiring and consequences for fruit quality traits. J. Exp. Bot. 2021, 72, 941–958. [Google Scholar] [CrossRef]
- Ciacka, K.; Staszek, P.; Sobczynska, K.; Krasuska, U.; Gniazdowska, A. Nitric Oxide in Seed Biology. Int. J. Mol. Sci. 2022, 23, 14951. [Google Scholar] [CrossRef]
- Parveen, N.; Kandhol, N.; Sharma, S.; Singh, V.P.; Chauhan, D.K.; Ludwig-Müller, J.; Corpas, F.J.; Tripathi, D.K. Auxin Crosstalk with Reactive Oxygen and Nitrogen Species in Plant Development and Abiotic Stress. Plant Cell Physiol. 2023, 63, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, P.; Tripathi, D.K.; Corpas, F.J.; Singh, V.P. Nitric oxide and hydrogen sulfide: An indispensable combination for plant functioning. Trends Plant Sci. 2021, 26, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Corpas, F.J. Crosstalk among hydrogen sulfide (H2S), nitric oxide (NO) and carbon monoxide (CO) in root-system development and its rhizosphere interactions: A gaseous interactome. Plant Physiol. Biochem. 2020, 155, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef]
- Mukherjee, S.; Corpas, F.J. H2O2, NO, and H2S networks during root development and signalling under physiological and challenging environments: Beneficial or toxic? Plant Cell Environ. 2023, 46, 688–717. [Google Scholar] [CrossRef]
- Corpas, F.J.; Palma, J.M. Functions of NO and H2S Signal Molecules against Plant Abiotic Stress. Methods Mol. Biol. 2023, 2642, 97–109. [Google Scholar]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Cañas, A.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 2018, 81, 36–45. [Google Scholar] [CrossRef]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. Inhibition of NADP-malic enzyme activity by H2S and NO in sweet pepper (Capsicum annuum L.) fruits. Physiol. Plant 2020, 168, 278–288. [Google Scholar] [CrossRef]
- González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Class III Peroxidases (POD) in Pepper (Capsicum annuum L.): Genome-Wide Identification and Regulation during Nitric Oxide (NO)-Influenced Fruit Ripening. Antioxidants 2023, 12, 1013. [Google Scholar] [CrossRef]
- González-Gordo, S.; López-Jaramillo, J.; Palma, J.M.; Corpas, F.J. Soybean (Glycine max L.) Lipoxygenase 1 (LOX 1) Is Modulated by Nitric Oxide and Hydrogen Sulfide: An In Vitro Approach. Int. J. Mol. Sci. 2023, 24, 8001. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, M.; Airaki, M.; Palma, J.M.; Chaki, M.; Barroso, J.B.; Corpas, F.J. Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 2012, 166, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Hu, Y.; Fan, T.; Li, J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci. Rep. 2015, 5, 8251. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Okamoto, M.; Crawford, N.M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Sciencie 2003, 302, 3–100. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, J.; An, R.; Zhang, J.; Liang, S.; Shao, J.; Liu, X.; An, L.; Yu, F. Mutations in circularly permuted GTPase family genes Atnoa1/RIF1/SVR10 and BPG2 suppress var2-mediated leaf variegation in Arabidopsis thaliana. Photosynth. Res. 2016, 127, 67–355. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B. Interaction of the hydrogen sulphide inhibitor, propargylglycine (PAG), with hydrogen sulphide on postharvest changes of the green leafy vegetable, pak choy. Postharvest Biol. Technol. 2019, 147, 54–58. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- He, X.-L.; Zhang, W.-Q.; Zhang, N.-N.; Wen, S.-M.; Chen, J. Hydrogen sulfide and nitric oxide regulate the adaptation to iron deficiency through affecting Fe homeostasis and thiol redox modification in Glycine max seedlings. Plant Physiol. Biochem. 2023, 194, 1–14. [Google Scholar] [CrossRef]
- Kharbech, O.; Sakouhi, L.; Ben Massoud, M.; Mur, L.A.; Corpas, F.J.; Djebali, W.; Chaoui, A. Nitric oxide and hydrogen sulfide protect plasma membrane integrity and mitigate chromium-induced methylglyoxal toxicity in maize seedlings. Plant Physiol. Biochem. 2020, 157, 244–255. [Google Scholar] [CrossRef]
- Kharbech, O.; Houmani, H.; Chaoui, A.; Corpas, F.J. Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J. Plant Physiol. 2017, 219, 71–80. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based Modification of Cysteine Desulfhydrase and the NADPH Oxidase RBOHD Controls Guard Cell Abscisic Acid Signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; González-Gordo, S.; Muñoz-Vargas, M.A.; Rodríguez-Ruiz, M.; Palma, J.M. The Modus Operandi of Hydrogen Sulfide(H2S)-Dependent Protein Persulfidation in Higher Plants. Antioxidants 2021, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Begara-Morales, J.C.; Chaki, M.; Sánchez-Calvo, B.; Mata-Pérez, C.; Leterrier, M.; Palma, J.M.; Barroso, J.B.; Corpas, F.J. Protein tyrosine nitration in pea roots during development and senescence. J. Exp. Bot. 2013, 64, 1121–1134. [Google Scholar] [CrossRef]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 68, 334–342. [Google Scholar] [CrossRef] [PubMed]
- González-Gordo, S.; Rodríguez-Ruiz, M.; López-Jaramillo, J.; Muñoz-Vargas, M.A.; Palma, J.M.; Corpas, F.J. Nitric Oxide (NO) Differentially Modulates the Ascorbate Peroxidase (APX) Isozymes of Sweet Pepper (Capsicum annuum L.) Fruits. Antioxidants 2022, 11, 765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).