Balance of Nitrate and Ammonium in Tropical Soil Conditions: Soil Factors Analyzed by Machine Learning
Abstract
1. Introduction
2. Material and Methods
2.1. Study Characterization
2.2. Data Analysis
3. Results and Discussion
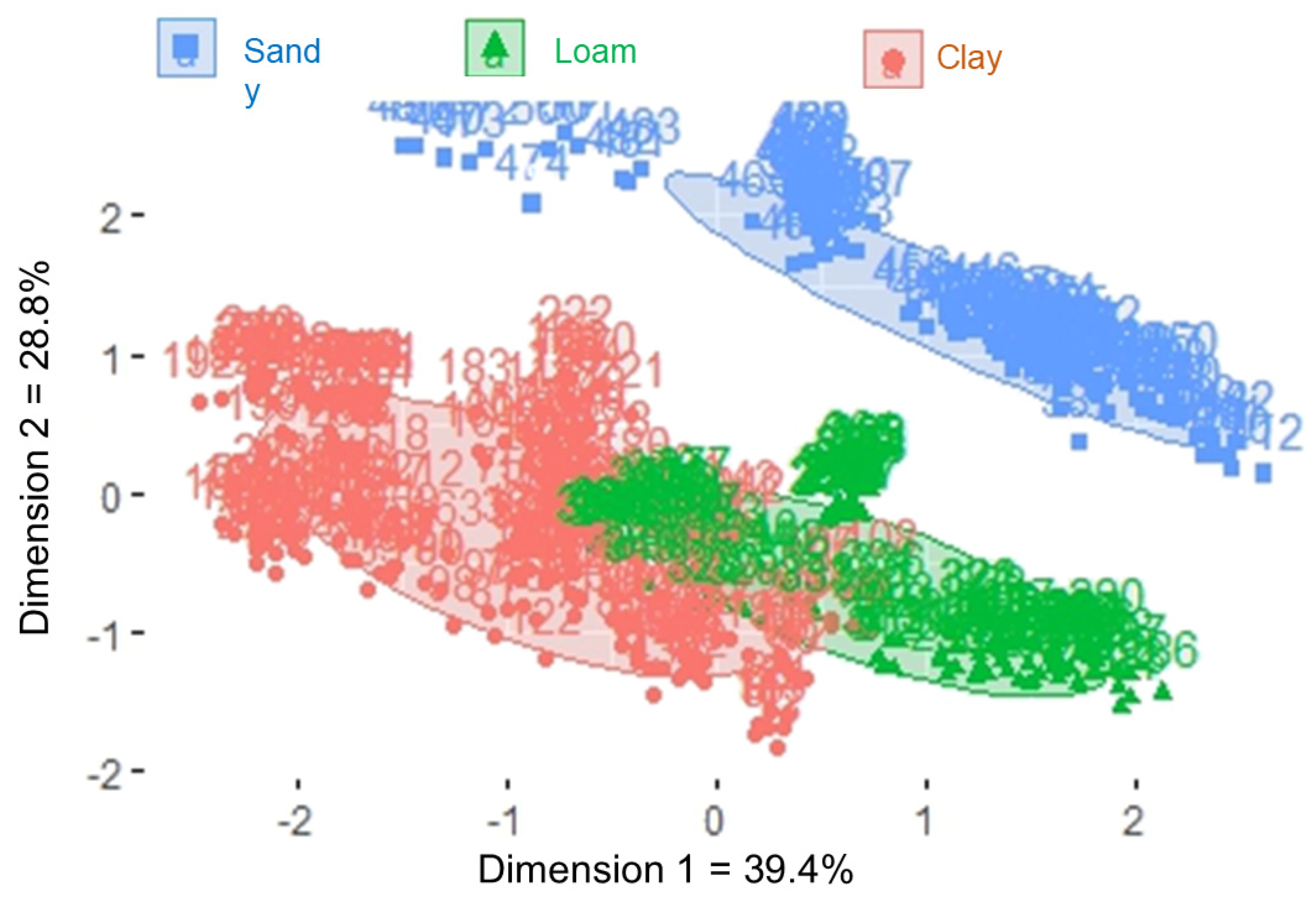
3.1. Cluster Formation
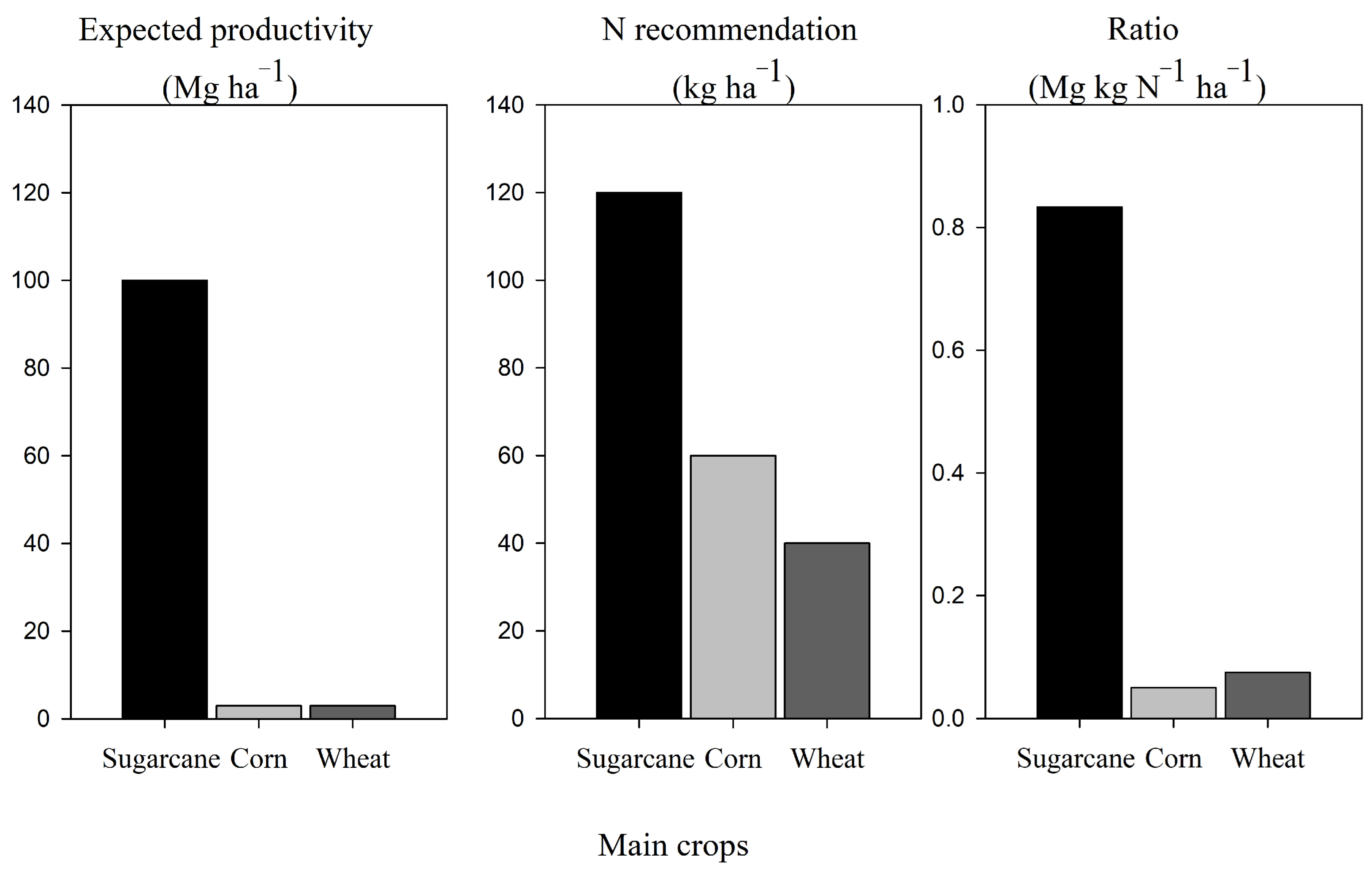
3.2. Expected and Recommended N
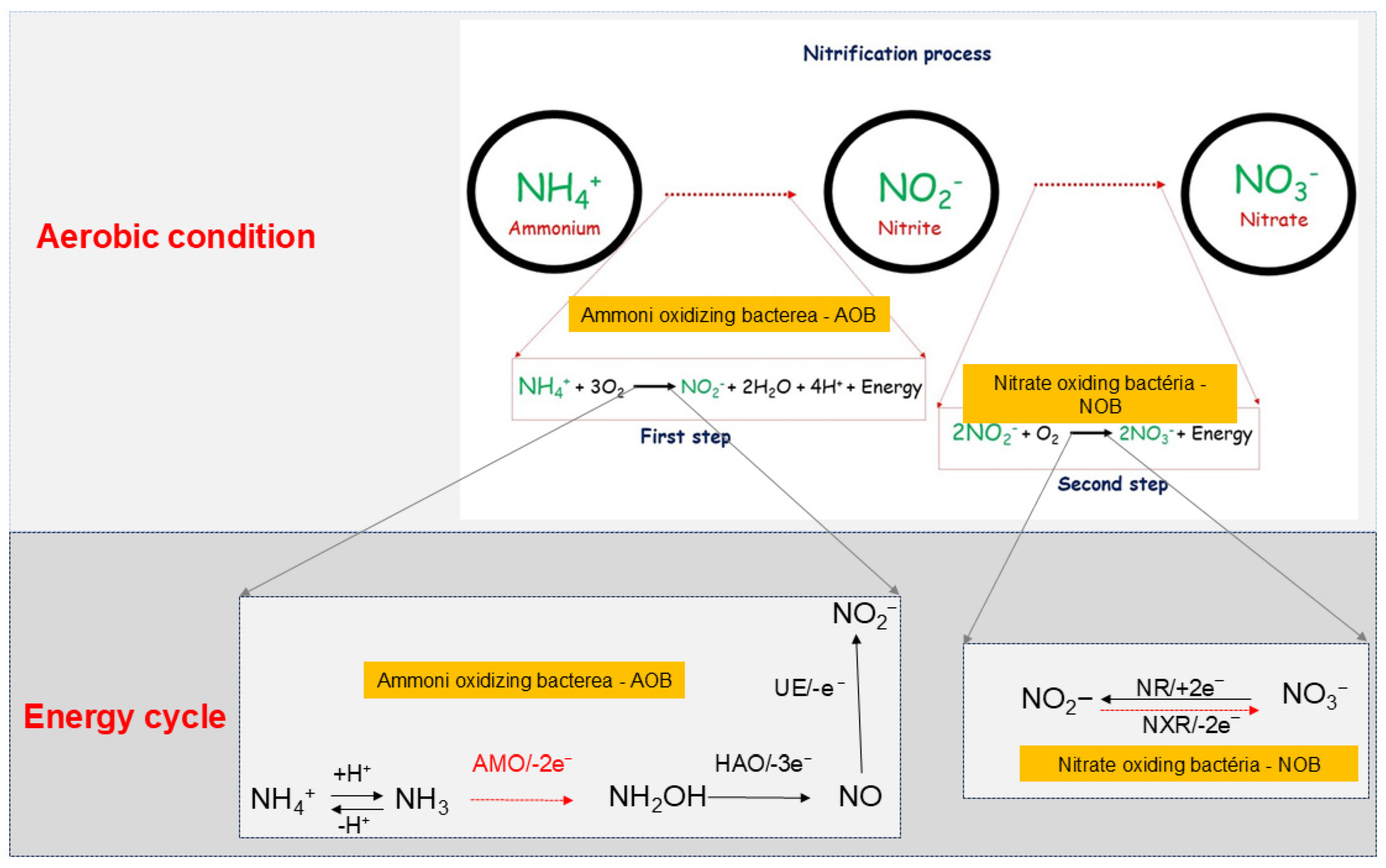
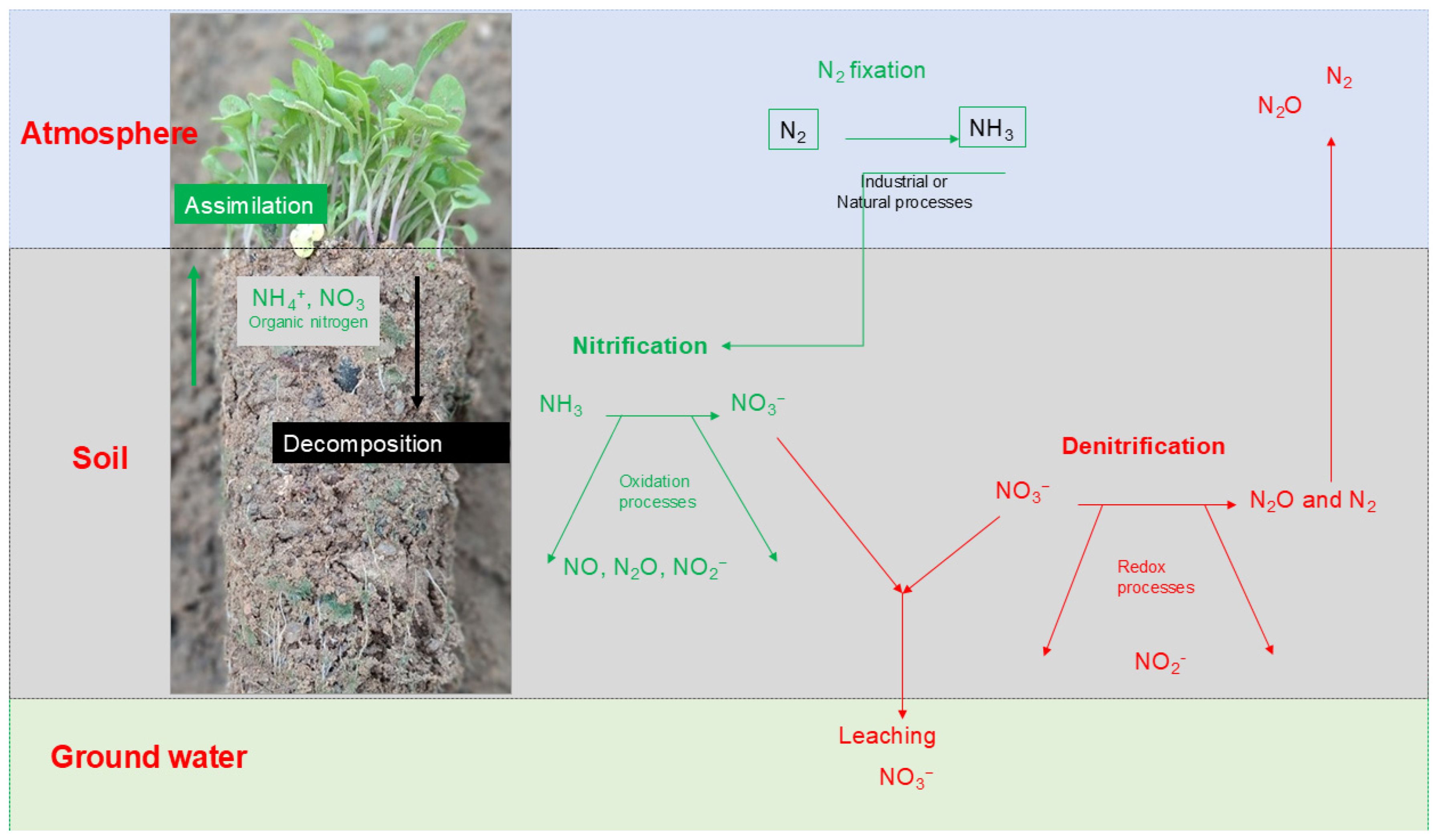
3.3. Routes of Nitrate and Ammonium in Soil
3.4. Soil Water on Nitrate and Ammonium Balance
3.5. Nitrate and Ammonium Contents in Agricultural Soil
3.6. Correlation between Soil Textures and the Processes of Nitrification
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Otto, R.; Netto, G.J.M.S.; Ferraz-Almeida, R.; Altarugio, L.M.; Favarin, J.L. Multisite response of sugarcane to nitrogen rates and split application under Brazilian field conditions. Agron. J. 2020, 113, 419–435. [Google Scholar] [CrossRef]
- Altarugio, L.M.; Savieto, J.; Machado, B.D.; Migliavacca, R.A.; Almeida, R.F.; Zavaschi, E.; Carneiro, L.D.; Vitti, G.C.; Otto, R. Optimal Management Practices for Nitrogen Application in Corn Cultivated During Summer and Fall in the Tropics. Commun. Soil Sci. Plant Anal. 2019, 50, 662–672. [Google Scholar] [CrossRef]
- Faria, I.K.; Vieira, J.L.; Tenelli, S.; Almeida, R.E.; Campos, L.J.; Costa, R.V.; Zavaschi, E.; Almeida, R.F.; Carneiro, L.D.; Otto, R. Optimal plant density and nitrogen rates for improving off-season corn yields in Brazil. Sci. Agric. 2019, 76, 344–352. [Google Scholar] [CrossRef]
- Sun, R.; Zhu, X.; Wang, C.; Yue, J.; Pan, L.; Song, C.; Zhao, Y. Effect of NH4+ and NO3− cooperatively regulated carbon to nitrogen ratio on organic nitrogen fractions during rice straw composting. Bioresour. Technol. 2024, 397, 130316. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. World Fertilizer Trends and Outlook to 2022; FAO: Rome, Italy, 2019. [Google Scholar]
- Gallucci, A.D.; Natera, M.; Moreira, L.A.; Nardi, K.T.; Altarugio, L.M.; de Mira, A.B.; de Almeida, R.F.; Otto, R. Nitrogen-Enriched Vinasse as a Means of Supplying Nitrogen to Sugarcane Fields: Testing the Effectiveness of N Source and Application Rate. Sugar Tech 2019, 21, 20–28. [Google Scholar] [CrossRef]
- USDA ERS. Fertilizer Use and Price; USDA ERS: Washington, DC, USA, 2020. Available online: https://www.ers.usda.gov/data-products/fertilizer-use-and-price/ (accessed on 1 August 2020).
- Biradar, S.S.; Patil, M.K.; Desai, S.A.; Singh, S.K.; Naik, V.R.; Lamani, K.; Joshi, A.K. Nitrogen use efficiency in bread wheat: Genetic variation and prospects for improvement. PLoS ONE 2024, 19, e0294755. [Google Scholar] [CrossRef]
- Ladha, J.K.; Tirol-Padre, A.; Reddy, C.K.; Cassman, K.G.; Verma, S.; Powlson, D.S.; Van Kessel, C.; de BRichter, D.; Chakraborty, D.; Pathak, H. Global nitrogen budgets in cereals: A 50-year assessment for maize, rice, and wheat production systems. Sci. Rep. 2016, 6, 19355. [Google Scholar] [CrossRef]
- Quassi de Castro, S.G.; Costa, V.E.; Quassi de Castro, S.A.; Carvalho, J.L.; Borges, C.D.; de Castro, R.A.; Kölln, O.T.; Franco, H.C. Fertilizer Application Method Provides an Environmental-Friendly Nitrogen Management Option for Sugarcane. J. Soil Sci. Plant Nutr. 2024, 24, 3195–3208. [Google Scholar] [CrossRef]
- Almeida, R.F.; Naves, E.R.; Silveira, C.H.; Wendling, B. Emissão de óxido nitroso em solos com diferentes usos e manejos: Uma revisão. Rev. Agronegocio Meio Ambiente 2015, 8, 441–461. [Google Scholar]
- Bruno, I.P.; Araújo, A.G.; Merten, G.H.; Ladeira, A.S.; Pinto, V.M. Crop Rotation and Nitrogen Fertilizer on Nitrate Leaching: Insights from a Low Rainfall Study. Nitrogen 2024, 5, 329–348. [Google Scholar] [CrossRef]
- Ferraz-Almeida, R.F.; Lopes, N.; Mota, R.; Wendling, B. Changing the sugarcane n credit and c fluxes in soil by the application of N-enhanced efficiency fertilizers. Compost Sci. Util. 2024, 31, 1–11. [Google Scholar] [CrossRef]
- Mikhael, J.E.R.; Almeida, R.F.; Franco, F.O.; Camargo, R.O.; Wendling, B. Recalcitrant carbon and nitrogen in agriculture soils with residue accumulation and fertilization under tropical conditions. Biosci. J. 2019, 35, 732–740. [Google Scholar] [CrossRef]
- Ferraz-Almeida, R.; Rodrigues Mikhael, J.E.; Oliveira Franco, F.; Fonseca Santana, L.M.; Wendling, B. Measuring the Labile and Recalcitrant Pools of Carbon and Nitrogen in Forested and Agricultural Soils: A Study under Tropical Conditions. Forests 2019, 10, 544. [Google Scholar] [CrossRef]
- Wendling, B.; Jucksch, I.; De Sá Mendonça, E.; De Almeida, R.F.; Alvarenga, R.C. Simulação dos estoques de Carbono e Nitrogênio pelo Modelo Century em Latossolos, no Cerrado Brasileiro. Rev. Cienc. Agron. 2014, 45, 238–248. [Google Scholar] [CrossRef][Green Version]
- de Almeida, R.F.; Silveira, C.H.; Mikhael, J.E.; Franco, F.O.; Ribeiro, B.T.; de Siqueira Ferreira, A.; de Sa Mendonca, E.; Wendling, B. CO2 emissions from soil incubated with sugarcane straw and nitrogen fertilizer. Afr. J. Biotechnol. 2014, 13, 3376–3384. [Google Scholar]
- Li, S.; Wang, X.; Hui, Z.; Miao, Y.; Li, S. qing. Soil Organic Nitrogen and Its Contribution to Crop Production. J. Integr. Agric. 2014, 13, 2061–2080. [Google Scholar] [CrossRef]
- Vilsmeier, K. Bestimmung von Dicyandiamid, Nitrit und Nitratin Bodenextrakten mit Hochdruckflüssigkeitschromatographie. Z. Pflanz. Bodenkunde 1984, 147, 264–268. [Google Scholar] [CrossRef]
- González-Pedraza, A.F.; Dezzeo, N. Effects of land use change and seasonality of precipitation on soil nitrogen in a dry tropical forest area in the western llanos of Venezuela. Sci. World J. 2014, 2014, 514204. [Google Scholar] [CrossRef] [PubMed]
- do Vale, D.W.; Prado, R.D.; Cantarella, H.; Fonseca, I.M.; Avalhães, C.C.; Correia, M.A.; Barbosa, E.M. Ammonium and nitrate in soil and ratoon sugarcane grown in function of nitrogen on oxisol. J. Plant Nutr. 2013, 36, 201–213. [Google Scholar] [CrossRef]
- Barth, G.; Otto, R.; Almeida, R.F.; Cardoso, E.J.; Cantarella, H.; Vitti, G.C. Conversion of ammonium to nitrate and abundance of ammonium-oxidizing-microorganism in tropical soils with nitrification inhibitor. Sci. Agric. 2020, 77, e20180370. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Santos, I.; Figueiredo, C.; Malaquias, J.; Santos Júnior, J.D.; de Carvalho, A.M. Nitrato e Amônio no solo sob Plantio Direto; Anais de congresso (CPAC): Luis Eduardo Magalhães, Brazil, 2014; pp. 1–7. [Google Scholar]
- Cantarella, H.; Quaggio, J.A.; Mattos, D.; Boareto, R.; Raij, B.V. Boletim 110, Recomendações de Adubação e Calagem para o Estado de São Paulo; Instituto Agronômico de Campinas: Campinas, Brazil, 2022. [Google Scholar]
- De Almeida, R.F.; Silveira, C.H.; Mota, R.P.; Moitinho, M.; Arruda, E.M.; Mendonça, E.D.; La Scala, N.; Wendling, B. For how long does the quality and quantity of residues in the soil affect the carbon compartments and CO2-C emissions? J. Soils Sediments 2016, 16, 2354–2364. [Google Scholar] [CrossRef]
- Ferraz-Almeida, R.; Lopes, S.N.; Wendling, B. How does N mineral fertilizer influence the crop residue N credit? Nitrogen 2020, 1, 99–110. [Google Scholar] [CrossRef]
- Risi, F.G.E.; Hüther, C.M.; Righi, C.A.; Umburanas, R.C.; Tezotto, T.; Dourado Neto, D.; Reichardt, K.; Pereira, C.R. Sustainability Analysis of Nitrogen Use Efficiency in Soybean-Corn Succession Crops of Midwest Brazil. Nitrogen 2024, 5, 232–253. [Google Scholar] [CrossRef]
- Torres, J.L.R.; Pereira, M.G. Production and decomposition residue culture preceding corn and soybeans in the savannah oxisol miner. Comun. Sci. 2014, 5, 419–426. [Google Scholar]
- Bolan, N.S.; Hedley, M.J.; White, R.E. Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil. 1991, 134, 53–63. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Y.; Zhang, K.; Xu, X.; Zhao, Y.; Bai, T.; Zhao, Y.; Wang, H.; Sheng, X.; Bloszies, S.; et al. Intermediate soil acidification induces highest nitrous oxide emissions. Nat. Commun. 2024, 15, 2695. [Google Scholar] [CrossRef]
- Rawal, A.; Lankau, R.A.; Ruark, M.D. How does soil organic matter affect potato productivity on sandy soil? Soil Sci. Soc. Am. J. 2024. early view. [Google Scholar] [CrossRef]
- Oliveira Junior, A.C.; Silva dos Santos, L.N.; Reis, M.N.O.; Vitorino, L.C.; Bessa, L.A.; Teixeira, M.B.; Soares, F.A.L. Effect of Mineral and Organic Nitrogen Sources on Vegetative Development, Nutrition, and Yield of Sugarcane. Agronomy 2023, 13, 1627. [Google Scholar] [CrossRef]
- Nyamangara, J.; Bergström, L.F.; Piha, M.I.; Giller, K.E. Fertilizer Use Efficiency and Nitrate Leaching in a Tropical Sandy Soil. J. Environ. Qual. 2003, 32, 599–606. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Chau, H.W.; Beare, M.; Cichota, R.; Teixeira, E.; Moore, T.; Di, H.; Cameron, K.; Guo, J.; et al. Response of nitrate leaching to no-tillage is dependent on soil, climate, and management factors: A global meta-analysis. Glob. Chang. Biol. 2023, 29, 2172–2187. [Google Scholar] [CrossRef] [PubMed]
- Zavaschi, E.; de Abreu Faria, L.; Ferraz-Almeida, R.; do Nascimento, C.A.; Pavinato, P.S.; Otto, R.; Vitti, A.C.; Vitti, G.C. Dynamic of P Flux in Tropical Acid Soils Fertilized with Humic Acid–Complexed Phosphate. J. Soil Sci. Plant Nutr. 2020, 20, 1937–1948. [Google Scholar] [CrossRef]
- Zavaschi, E.; Ferraz-Almeida, R.; Fária, L.A.; Otto, R.; Vitti, A.C.; Vitti, G.C. Application of superphosphate complexed with humic acid in an area of sugarcane. Rev. Ciênc. Agron. 2020, 51, e20186463. [Google Scholar] [CrossRef]
- Mahmud, K.; Panday, D.; Mergoum, A.; Missaoui, A. Nitrogen Losses and Potential Mitigation Strategies for a Sustainable Agroecosystem. Sustainability 2021, 13, 2400. [Google Scholar] [CrossRef]
- IPCC. Overview 2 2019 IPCC Guidelines for National Greenhouse Gas Inventories. 2019. Available online: https://www.ipcc.ch/report/2019-refinement-to-the-2006-ipcc-guidelines-for-national-greenhouse-gas-inventories/ (accessed on 1 August 2020).
- Maraseni, T.N.; Qu, J. An international comparison of agricultural nitrous oxide emissions. J. Clean. Prod. 2016, 135, 1256–1266. [Google Scholar] [CrossRef]
- Kostyanovsky, K.I.; Huggins, D.R.; Stockle, C.O.; Morrow, J.G.; Madsen, I.J. Emissions of N2O and CO2 Following Short-Term Water and N Fertilization Events in Wheat-Based Cropping Systems. Front. Ecol. Evol. 2019, 7, 63. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Joris, H.A.W.; Vitti, A.C.; Ferraz-Almeida, R.; Otto, R.; Cantarella, H. Long-term N fertilization reduces uptake of N from fertilizer and increases the uptake of N from soil. Sci. Rep. 2020, 10, 18834. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen: A global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef]
- Qiao, N.; Xu, X.; Hu, Y.; Blagodatskaya, E.; Liu, Y.; Schaefer, D.; Kuzyakov, Y. Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Sci. Rep. 2016, 6, 19865. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Shao, R.; Du, W.; Liu, Y.H. Soil priming effect mediated by nitrogen fertilization gradients in a semi-arid grassland, China. J. Resour. Ecol. 2019, 10, 147. [Google Scholar]
- Ma, H.L.; Gao, R.; Yin, Y.F.; Yang, Y.S. Effects of leaf litter tannin on soil ammonium and nitrate content in two different forest soils of mount Wuyi, China. Toxicol. Environ. Chem. 2016, 98, 395–409. [Google Scholar] [CrossRef]
- Gurmesa, G.A.; Li, S.; Zhu, W.; Gundersen, P.; Zhang, S.; Xi, D.; Huang, S.; Wang, A.; Jiang, Y.; Zhu, J.; et al. Fate of atmospherically deposited NH4+ and NO3 in twotemperate forests in China: Temporal pattern and redistribution. Ecol. Appl. 2019, 29, e01920. [Google Scholar]
- Tills, A.R.; Alloway, B.J. The effect of ammonium and nitrate nitrogen sources on copper uptake and amino acid status of cereals. Plant Soil 1981, 62, 279–290. [Google Scholar] [CrossRef]
- Andriolo, J.L.; Godoi, R.D.; Cogo, C.M.; Bortolotto, O.C.; da Luz, G.L.; Madaloz, J.C. Growth and development of lettuce plants at high NH4+:NO3− ratios in the nutrient solution. Hortic. Bras. 2006, 24, 352–355. [Google Scholar] [CrossRef][Green Version]
- Fan, Z.; Lali, M.N.; Xiong, H.; Luo, Y.; Wang, Y.; Wang, Y.; Lu, M.; Wang, J.; He, X.; Shi, X.; et al. Seedlings of Poncirus trifoliata exhibit tissue-specific detoxification in response to NH4+ toxicity. Plant Biol. 2024, 26, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, Y.; Zhang, B.; Li, H.; Wang, Z.; Si, J.; Fan, S.; Feng, B. Does energy cost constitute the primary cause of ammonium toxicity in plants? Plant 2022, 256, 62. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Viciedo, D.; Salas Aguilar, D.; de Mello Prado, R.; Peña Calzada, K.; Calero Hurtado, A.; de Cássia Piccolo, M.; Bomfim Soares, M.; Lizcano Toledo, R.; Alves, G.R.; Ferreira, D.; et al. Silicon-Mediated Adjustments in C:N:P Ratios for Improved Beetroot Yield under Ammonium-Induced Stress. Agronomy 2024, 14, 1104. [Google Scholar] [CrossRef]
- Koltun, A.; Maniero, R.A.; Vitti, M.; de Setta, N.; Giehl, R.F.H.; Lima, J.E.; Figueira, A. Functional characterization of the sugarcane (Saccharum spp.) ammonium transporter AMT2;1 suggests a role in ammonium root-to-shoot translocation. Front. Plant Sci. 2022, 13, 1039041. [Google Scholar] [CrossRef]
- Rashti, M.R.; Nelson, P.N.; Lan, Z.; Su, N.; Esfandbod, M.; Liu, X.; Goloran, J.; Zhang, H.; Chen, C. Sugarcane cultivation altered soil nitrogen cycling microbial processes and decreased nitrogen bioavailability in tropical Australia. J. Soils Sediments 2024, 24, 946–955. [Google Scholar] [CrossRef]
- Boschiero, B.N.; Mariano, E.; Azevedo, R.A.; Ocheuze Trivelin, P.C. Influence of nitrate—Ammonium ratio on the growth, nutrition, and metabolism of sugarcane. Plant Physiol. Biochem. 2019, 139, 246–255. [Google Scholar] [CrossRef]
- Robinson, N.; Brackin, R.; Vinall, K.; Soper, F.; Holst, J.; Gamage, H.; Paungfoo-Lonhienne, C.; Rennenberg, H.; Lakshmanan, P.; Schmidt, S. Nitrate paradigm does not hold up for sugarcane. PLoS ONE 2011, 6, 19045. [Google Scholar] [CrossRef] [PubMed]
- Boschiero, B.N.; Mariano, E.; Trivelin, P.C.O. “Preferential” ammonium uptake by sugarcane does not increase the 15N recovery of fertilizer sources. Plant Soil 2018, 429, 253–269. [Google Scholar] [CrossRef]
- Furtado da Silva, N.; Cabral da Silva, E.; Muraoka, T.; Batista Teixeira, M.; Antonio Loureiro Soares, F.; Nobre Cunha, F.; Adu-Gyamfi, J.; Cavalcante, W.S. Nitrogen Utilization from Ammonium Nitrate and Urea Fertilizer by Irrigated Sugarcane in Brazilian Cerrado Oxisol. Agriculture 2020, 10, 323. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Manzano-Agugliaro, F. Global trends in nitrate leaching research in the 1960–2017 period. Sci. Total Environ. 2018, 643, 400–413. [Google Scholar] [CrossRef]
- Bortolotto, R.P.; Bruno, I.P.; Dourado-Neto, D.; Timm, L.C.; Silva, A.N.; Reichardt, K. Nitrate leaching through climatologic water balance in a fertigated coffee plantation. Rev. Ceres 2013, 60, 785–792. [Google Scholar] [CrossRef]



| Soil Textures | Average | Minimum | Maximum | Variation, % |
|---|---|---|---|---|
| NH4+, mg kg−1 | ||||
| Clay | 31.0 | 24.9 | 207.7 | ±182.8 (±88%) |
| Loam | 46.2 | 35.2 | 155.0 | ±119.7 (±77%) |
| Sandy | 57.0 | 2.7 | 135.0 | ±132.2 (±98%) |
| NO3−, mg kg−1 | ||||
| Clay | 104.2 | 5.1 | 104.0 | ±98.9 (±95%) |
| Loam | 94.5 | 6.8 | 108.3 | ±101.5 (±96%) |
| Sandy | 36.4 | 4.7 | 103.2 | ±98.5 (±95%) |
| Soil pH | ||||
| Clay | 5.9 | 0.1 | 6.6 | ±6.4 (±92%) |
| Loam | 7.0 | 0.1 | 7.6 | ±7.5 (±91%) |
| Sandy | 5.9 | 0.1 | 6.8 | ±6.8 (±91%) |
| Cluster results | ||||
| Cluster 1 | Cluster 2 | Cluster 3 | ||
| NH4+ | −0.25 | 0.12 | 0.39 | |
| NO3− | 0.39 | 0.19 | −0.99 | |
| pH | −0.46 | 1.4 | −0.47 | |
| Sum of squares | 578.24 | 232.21 | 237.77 | |
| Number | 252 | 126 | 126 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz-Almeida, R. Balance of Nitrate and Ammonium in Tropical Soil Conditions: Soil Factors Analyzed by Machine Learning. Nitrogen 2024, 5, 732-745. https://doi.org/10.3390/nitrogen5030048
Ferraz-Almeida R. Balance of Nitrate and Ammonium in Tropical Soil Conditions: Soil Factors Analyzed by Machine Learning. Nitrogen. 2024; 5(3):732-745. https://doi.org/10.3390/nitrogen5030048
Chicago/Turabian StyleFerraz-Almeida, Risely. 2024. "Balance of Nitrate and Ammonium in Tropical Soil Conditions: Soil Factors Analyzed by Machine Learning" Nitrogen 5, no. 3: 732-745. https://doi.org/10.3390/nitrogen5030048
APA StyleFerraz-Almeida, R. (2024). Balance of Nitrate and Ammonium in Tropical Soil Conditions: Soil Factors Analyzed by Machine Learning. Nitrogen, 5(3), 732-745. https://doi.org/10.3390/nitrogen5030048







