Estimating Nitrogen Uptake Efficiency of Mango Varieties from Foliar KNO3 Application Using a 15N Tracer Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Seedling Preparation
| Variety | Parentage | Origin | Fruit Characteristics | Other Features | References |
|---|---|---|---|---|---|
| ‘KP’ | Unknown | Queensland, Australia | Ripens to a rich yellow with bright pink shoulders; sweet, low-fiber, medium-sized fruit; and preferred for its distinctive taste. | Polyembryonic, irregular bearing, high vigor tree | [35] |
| ‘B74’ | ‘Sensation’ x ‘KP’ | Queensland, Australia | Ripens to a yellow skin color with bright red shoulder; sweet with mild ‘KP’ flavor; firm flesh and free of fiber; medium-sized fruit. | Monoembryonic, consistent bearing, medium vigor tree | [35] |
| ‘NMBP 1201’ | ‘Irwin’ x ‘KP’ | Queensland, Australia | Ripens to a yellow background skin with a soft pink blush; rich in ‘KP’ flavor; yellow orange; soft texture, firm, very-low-fiber flesh | Monoembryonic, with tendency of biennial bearing, medium vigor, compact tree canopy | [36] |
| ‘NMBP 1243’ | ‘Irwin’ x ‘KP’ | Queensland, Australia | Ripens to a yellow background skin with a strong red/pink blush; a classical ‘KP’ flavor; light orange; soft texture, with firm, very slight fiber flesh | Monoembryonic, medium vigor, open tree canopy | [36] |
| ‘NMBP 4069’ | ‘Van Dyke’ x ‘KP’ | Queensland, Australia | Ripens to a yellow background skin with a soft pink blush; sweet and rich in ‘KP’ flavor; yellow/orange; soft texture, with firm, low-fiber flesh | Monoembryonic, with tendency of biennial bearing, medium vigor tree | [36] |
2.2. Treatments and Experimential Design
2.3. Sample Preparation and Calculation
2.4. Statistical Analysis
3. Results
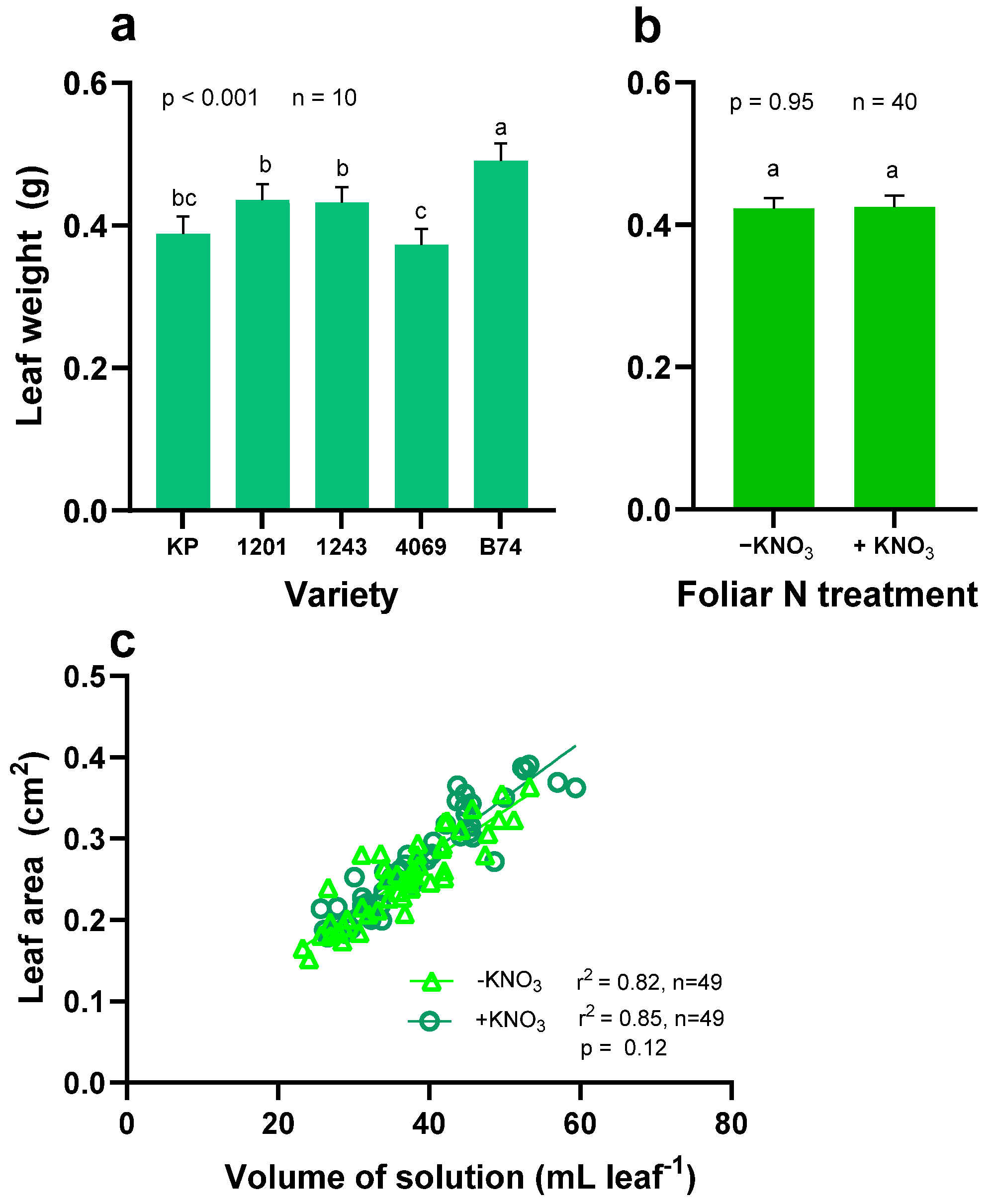
3.1. Leaf Weight, Leaf Area, and Volume of Solution Taken by the Leaves
3.2. Leaf Atom % 15N Content
3.3. Ndff and NUpE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 18 July 2024).
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and principles of environmentally sustainable food and agriculture systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; van Vianen, J.; Foli, S.; Clendenning, J.; Yang, K.; MacDonald, M.; Petrokofsky, G.; Padoch, C.; Sunderland, T. Trees for life: The ecosystem service contribution of trees to food production and livelihoods in the tropics. For. Policy Econ. 2017, 84, 62–71. [Google Scholar] [CrossRef]
- Demestihas, C.; Plénet, D.; Génard, M.; Raynal, C.; Lescourret, F. Ecosystem services in orchards. A review. Agron. Sustain. Dev. 2017, 37, 12. [Google Scholar] [CrossRef]
- Mangoes in Western Australia, Department of Primary Industry and Regional Development, Government of Western Australia. Available online: https://www.agric.wa.gov.au/mangoes/mangoes-western-australia (accessed on 7 December 2024).
- Hort Innovation. Australian Horticulture Statistics Handbook; Hort Innovation: Sydney, NSW, Australia, 2024; pp. 100–103. Available online: https://www.horticulture.com.au/globalassets/hort-innovation/australian-horticulture-statistics-handbook/ort-stats-intro-22-23.pdf (accessed on 7 December 2024).
- NT Mangoes, Northern Territory, Australia. Available online: https://ntfarmers.org.au/commodities/mangoes/ (accessed on 28 June 2024).
- Bally, I.S.E.; Ibell, P.T. Improvement of mango tree architecture. Acta Hortic. 2015, 1075, 59–64. [Google Scholar] [CrossRef]
- Hamilton, D.; Martin, C.; Bennet, M.; Hearnden, M.; Asis, C.A. Effect of tree leaf N status and N application time on yield and fruit N partitioning of mango. Acta Hortic. 2017, 1183, 161–166. [Google Scholar] [CrossRef]
- Davenport, T.L. Reproductive physiology. In The Mango: Botany Production and Uses, 2nd ed.; Litz, R.E., Ed.; CAB International: Wallingford, UK, 2009; pp. 97–169. [Google Scholar]
- Understanding Mango Flowering in Darwin, Northern Territory Department of Industry, Tourism and Trade, Australia. Available online: https://www.industry.mangoes.net.au/cmsb/media/nt-flowering-factsheet_upload.pdf (accessed on 15 June 2024).
- Bangerth, K.F. Floral induction in mature, perennial angiosperm fruit trees: Similarities and discrepancies with annual/biennial plants and the involvement of plant hormones. Sci. Hortic. 2009, 122, 153–163. [Google Scholar] [CrossRef]
- Sarkhosh, A.; McConchie, C.; Khadivi, A. The effects of different tip-pruning times on flowering, yield, and maturity of two mango cultivars in subtropical climate of Northern Territory (Katherine region) from Australia. Sci. Hortic. 2018, 234, 140–145. [Google Scholar] [CrossRef]
- McConchie, C.; Manipulating Mango Flowering Extend Harvest Window. Hort Innovation, Sydney, Australia. Available online: https://www.horticulture.com.au/globalassets/laserfiche/assets/project-reports/mg12012/mg12012---final-report-complete.pdf (accessed on 20 June 2024).
- Fernández, V.; Bahamonde, H.A.; Javier Peguero-Pina, J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar water and solute absorption: An update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.-P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 5293–5306. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Nájera-González, H.R.; Nigam, D.; Khan, A.; Chen, J.; Xin, Z.; Herrera-Estrella, L.; Jiao, Y. Leucine-rich repeat receptor kinase BM41 regulates cuticular wax deposition in sorghum. J. Exp. Bot. 2024, 75, 6331–6345. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef]
- Eichert, T.; Fernandez, V. Uptake and release of mineral elements by leaves and other aerial plant parts. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 71–84. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Silber, A.; Goldberg, T.; Shapira, O.; Hochberg, U. Nitrogen uptake and macronutrients distribution in mango (Mangifera indica L. cv. Keitt) trees. Plant Physiol. Biochem. 2022, 181, 23–32. [Google Scholar] [CrossRef]
- Ramírez, F.; Davenport, T.L. Mango (Mangifera indica L.) flowering physiology. Sci. Hortic. 2010, 162, 65–72. [Google Scholar] [CrossRef]
- Sudha, R.; Balamohan, T.N.; Soorianathasundaram, K. Effect of foliar spray of nitrogenous chemicals on flowering, fruit set and yield in mango (Mangifera indica L.) cv. Alphonso. J. Hortic. Sci. 2012, 7, 190–193. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef]
- Shah, I.H.; Jinhui, W.; Li, X.; Hameed, M.K.; Manzoor, M.A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Exploring the role of nitrogen and potassium in photosynthesis implications for sugar: Accumulation and translocation in horticultural crops. Sci. Hortic. 2024, 327, 112832. [Google Scholar] [CrossRef]
- Biggest Planting of New Mango Varieties Yess! AhHa! and Now! by Manbulloo in Northern Territory. Available online: https://www.abc.net.au/news/2024-05-28/biggest-patch-new-mango-varieties-yess-ahha-now-planted-in-nt/103888440?utm_campaign=abc_news_web&utm_content=link&utm_medium=content_shared&utm_source=abc_news_web (accessed on 10 June 2024).
- Scandellari, F.; Tagliavini, M. The use of the 15N stable isotope technique to improve the management of nitrogen nutrition of fruit trees-a mini review. Acta Hortic. 2017, 1217, 191–200. [Google Scholar] [CrossRef]
- Meurant, N.; Holmes, R.; MacLeod, N.; Fullelove, G.; Bally, I.S.; Kernot, I. Mango Information Kit. Agrilink, Your Growing Guide to Better Farming Guide. 1999. Available online: https://era.daf.qld.gov.au/id/eprint/1647/ (accessed on 8 August 2024).
- Australian National Mango Breeding Program, Department of Primary Industry and Regional Development, Government of Western Australia. Available online: https://www.agric.wa.gov.au/mangoes/australian-national-mango-breeding-program (accessed on 8 August 2024).
- Donovan, N.; Bally, I.; Cooke, T. Nursery Manual for Citrus and Mango; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 2016; pp. 82–91. [Google Scholar]
- Loveland Industries LI 700 Surfactant. Loveland Products. Available online: https://www.lovelandagriproducts.com.au/adjuvants/leci-tech/li-700 (accessed on 10 March 2024).
- Asis, C.A.; Alexander, T.; Sarkhosh, A.; Umar, M.; McConchie, C. Optimising foliar nitrogen uptake of mango: Effect of adjuvant, leaf position and time of potassium nitrate spray. Acta Hortic. 2020, 1299, 269–274. [Google Scholar] [CrossRef]
- Silcox, S.; Holloway, P.J. A simple method for the removal of foliar deposits of agrochemicals using cellulose acetate film stripping. Aspects Appl. Biol. 1986, 11, 13–17. [Google Scholar]
- Scott: Automation That Transforms. Available online: https://scottautomation.com/en/ (accessed on 23 November 2024).
- IAEA. Application of nuclear techniques in soil fertility and plant nutrition studies. In Use of Isotope and Radiation Methods in Soil and Water Management and Crop Nutrition—Training Course Series No. 14; International Atomic Energy Agency: Vienna, Austria, 2001; Chapter 2; pp. 21–103. [Google Scholar]
- Htwe, N.M.; Phyu, S.L.; Thu, C.N. Assessment of genetic variability and character association of Myanmar local rice (Oryza sativa L.) germplasm. J. Exp. Agric. Int. 2019, 40, 1–10. [Google Scholar] [CrossRef]
- GraphPad: Comprehensive Analysis and Powerful Statistics, Simplified. Available online: https://www.graphpad.com/scientific-software/prism/)/ (accessed on 23 November 2024).
- Nevin, J.M.; Embleton, T.W.; Lovatt, C.J. Problems with urea-N foliar fertilization of avocado. Acta Hortic. 1989, 275, 535–542. [Google Scholar] [CrossRef]
- Lu, P.; Chacko, E.K.; Bithell, S.L.; Schaper, H.; Wiebel, J.; Cole, S.; Müller, W.J. Photosynthesis and stomatal conductance of five mango cultivars in the seasonally wet and dry tropics of northern Australia. Sci. Hortic. 2012, 138, 108–119. [Google Scholar] [CrossRef]
- Tanou, G.; Ziogast, V.; Molassiotis, A. Foliar nutrition, biostimulants and prime-like dynamics in fruit tree physiology: New insights on an old topic. Front. Plant Sci. 2017, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Bright, J.; McAlister, S.; Renfree, R. Foliar Nitrogen Nutrition of Mango; Technical Annual Report 1998–1999; Department of Industry and Forestry: Darwin, Northern Territory, Australia, 2000; p. 109.
- Ram, R.A.; Rahim, M.A.; Alam, M.S. Diagnosis and management of nutrient constraints in mango. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 12, pp. 629–650. [Google Scholar] [CrossRef]
- Saúco, V.G. Advances in mango cultivation. In Achieving Sustainable Cultivation of Tropical Fruits, 1st ed.Yahia, E.M., Ed.; Burleigh Dodds Science Publishing: Philadelphia, PA, USA, 2019; pp. 489–518. [Google Scholar]
- Nguyen, H.; Hofman, P.; Holmes, R.; Bally, I.; Stubbingsy, B.; McConchie, R. Effect of nitrogen on the skin colour and other quality attributes of ripe Kensington Pride mango (Mangifera indica L.) fruit. J. Hort. Sci. Biotechnol. 2004, 79, 204–210. [Google Scholar] [CrossRef]
- Asis, C.A.; Tilbrook, J.; Anson, D.; Niscioli, A.; Guinto, D.; Bristow, M.; Rowlings, D. Nitrogen level impacting fruit yield and quality of mango in northern tropical Australia. Sustainability, 2024; submitted. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asis, C.A.; Tilbrook, J.; Anson, D.; Niscioli, A.; Bristow, M.; Friedl, J.; Rowlings, D. Estimating Nitrogen Uptake Efficiency of Mango Varieties from Foliar KNO3 Application Using a 15N Tracer Technique. Nitrogen 2024, 5, 1124-1134. https://doi.org/10.3390/nitrogen5040072
Asis CA, Tilbrook J, Anson D, Niscioli A, Bristow M, Friedl J, Rowlings D. Estimating Nitrogen Uptake Efficiency of Mango Varieties from Foliar KNO3 Application Using a 15N Tracer Technique. Nitrogen. 2024; 5(4):1124-1134. https://doi.org/10.3390/nitrogen5040072
Chicago/Turabian StyleAsis, Constancio A., Joanne Tilbrook, Dallas Anson, Alan Niscioli, Mila Bristow, Johannes Friedl, and David Rowlings. 2024. "Estimating Nitrogen Uptake Efficiency of Mango Varieties from Foliar KNO3 Application Using a 15N Tracer Technique" Nitrogen 5, no. 4: 1124-1134. https://doi.org/10.3390/nitrogen5040072
APA StyleAsis, C. A., Tilbrook, J., Anson, D., Niscioli, A., Bristow, M., Friedl, J., & Rowlings, D. (2024). Estimating Nitrogen Uptake Efficiency of Mango Varieties from Foliar KNO3 Application Using a 15N Tracer Technique. Nitrogen, 5(4), 1124-1134. https://doi.org/10.3390/nitrogen5040072






