Inoculation with Bradyrhizobium elkanii Reduces Nitrogen Fertilization Requirements for Pseudalbizzia niopoides, a Multipurpose Neotropical Legume Tree
Abstract
1. Introduction
2. Materials and Methods
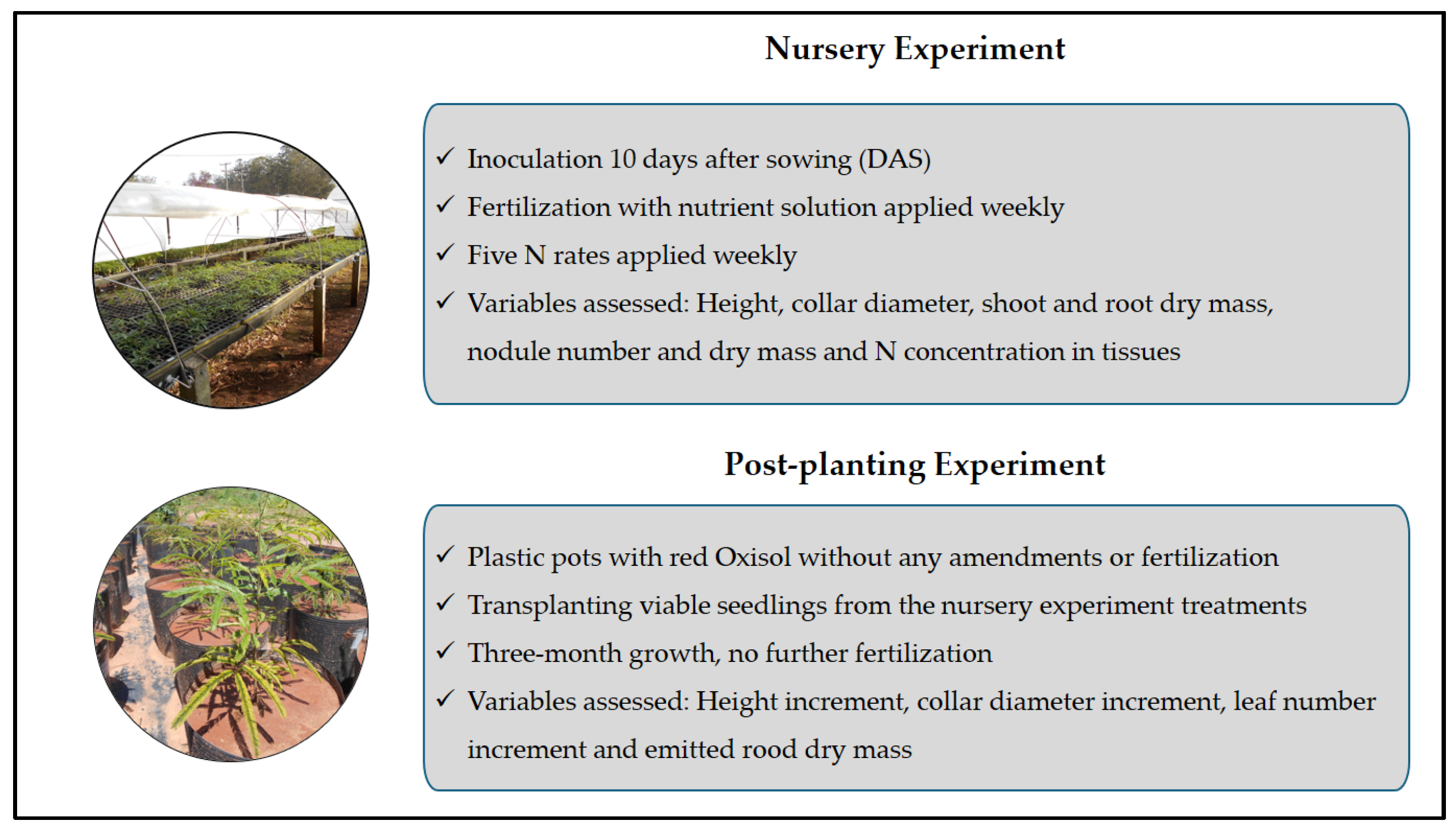
2.1. Nursery Experiment
2.2. Post-Planting Experiment
2.3. Statistical Analysis
3. Results
3.1. Nonlinear Regression Models for Nursery Experiment
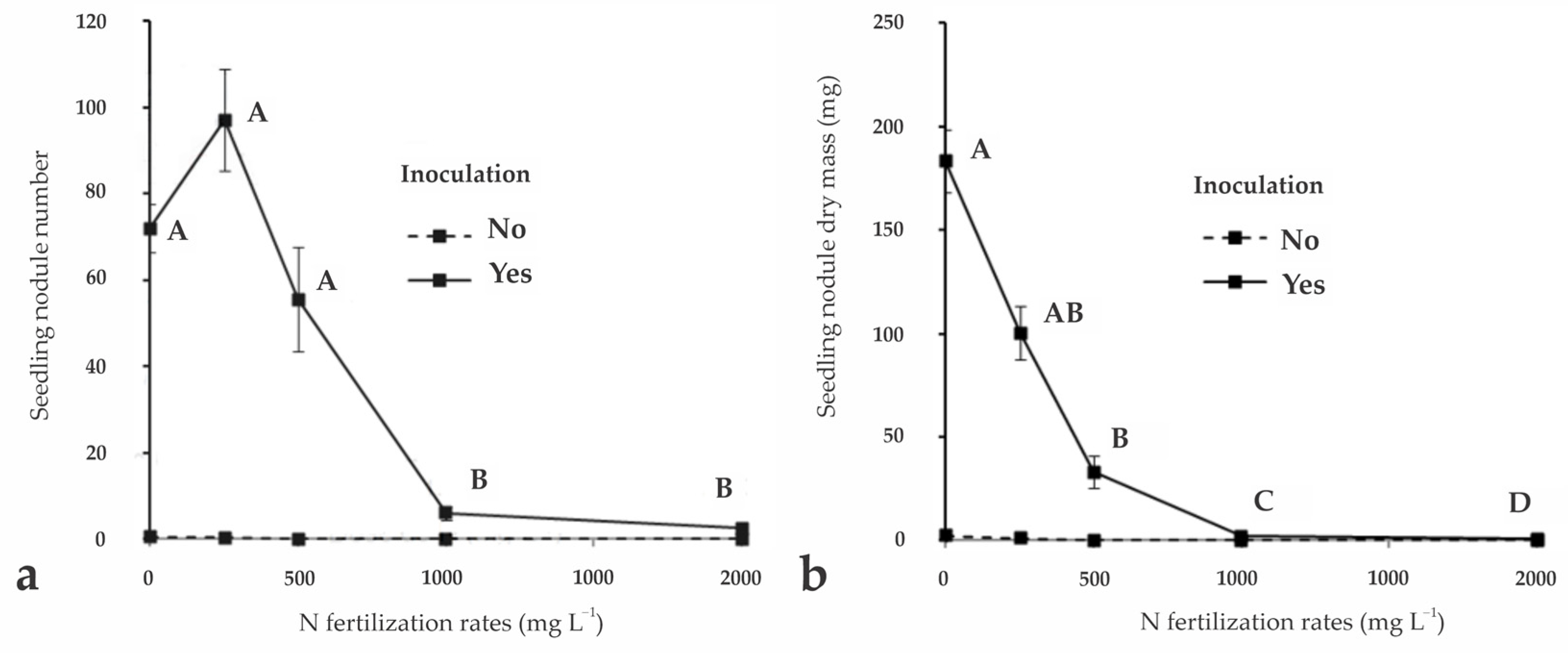
3.2. Generalized Linear Models for Nursery Experiment
3.3. Generalized Linear Models for Pot Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.F.; Wang, E.T.; Ji, Z.J.; Zhang, J.J. Recent development and new insight of diversification and symbiosis specificity of legume rhizobia: Mechanism and application. J. Appl. Microbiol. 2021, 131, 553–563. [Google Scholar] [CrossRef]
- Sousa, W.S.; Soratto, R.P.; Peixoto, D.S.; Campos, T.S.; Silva, M.B.; Souza, A.G.V.; Teixeira, I.R.; Gitari, H.I. Effects of Rhizobium Inoculum compared with mineral nitrogen fertilizer on nodulation and seed yield of Common Bean. A Meta-Analysis. Agron. Sustain. Dev. 2022, 42, 52. [Google Scholar] [CrossRef]
- Teixeira, H.; Rodríguez-Echeverría, S. Identification of symbiotic Nitrogen-Fixing Bacteria from three African leguminous trees in Gorongosa National Park. Syst. Appl. Microbiol. 2016, 39, 350–358. [Google Scholar] [CrossRef]
- Xu, H.; Detto, M.; Li, Y.; Li, Y.; He, F.; Fang, S. Do N-fixing legumes promote neighbouring diversity in the Tropics? J. Ecol. 2019, 107, 229–239. [Google Scholar] [CrossRef]
- Makoto, K.; Utsumi, S.; Zeng, R.; Mamiya, W.; Miyazaki, T.; Okuyama, T.; Tanaka, F.; Yamada, T.; Yoshida, t. which native legume or non-legume Nitrogen-Fixing Tree is more efficient in restoring post-landslide forests along an environmental gradient? For. Ecol. Manag. 2024, 554, 121672. [Google Scholar] [CrossRef]
- Bakhoum, N.; Odee, D.W.; Fall, D.; Ndoye, F.; Kane, A.; Kimiti, J.M.; Zoubeirou, A.M.; Sylla, S.N.; Noba, K.; Diouf, D. Senegalia senegal response to inoculation with rhizobial strains vary in relation to seed provenance and soil type. Plant Soil 2016, 398, 181–193. [Google Scholar] [CrossRef][Green Version]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012. [Google Scholar]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume Nodulation: The host controls the party. Plant Cell Environ. 2019, 42, 41–51. [Google Scholar] [CrossRef]
- Tasca, H.C.; Posso, D.A.; Mossi, A.J.; Bayer, C.; Cansian, R.L.; Chavarria, G.; Sausen, T.L. Impact of nodulation efficiency and concentrations of soluble sugars and ureides on Soybean water deficit during vegetative growth. Nitrogen 2024, 5, 992–1000. [Google Scholar] [CrossRef]
- Dianda, M.; Chalifour, F.-P. Effets du N minéral et du génotype de la plante sur la croissance et la nodulation de Faidherbia albida. Can. J. Bot. 2002, 80, 241–254. [Google Scholar] [CrossRef]
- Kuykendall, L.D.; Saxena, B.; Devine, T.E.; Udell, S.E. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp.nov. Can. J. Microbiol. 1992, 38, 501–505. [Google Scholar] [CrossRef]
- Leng, P.; Jin, F.; Li, S.; Huang, Y.; Zhang, C.; Shan, Z.; Yang, Z.; Chen, L.; Cao, D.; Hao, Q.; et al. High efficient broad-spectrum Bradyrhizobium elkanii Y63-1. Oil Crop Sci. 2023, 8, 228–235. [Google Scholar] [CrossRef]
- Nguyen, H.P.; Ratu, S.T.N.; Yasuda, M.; Teaumroong, N.; Okazaki, S. Identification of Bradyrhizobium elkanii USDA61 Type III effectors determining symbiosis with Vigna mungo. Genes 2020, 11, 474. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.S.; Nóbrega, R.S.A.; Barberi, A.; Da Silva, K.; Ferreira, D.F.; Moreira, F.M.D.S. Nitrogen-Fixing Bacteria communities occurring in soils under different uses in the Western Amazon Region as indicated by nodulation of Siratro (Macroptilium atropurpureum). Plant Soil 2009, 319, 127–145. [Google Scholar] [CrossRef]
- Binde, D.R.; Menna, P.; Bangel, E.V.; Barcellos, F.G.; Hungria, M. Rep-PCR Fingerprinting and taxonomy based on the sequencing of the 16S rRNA gene of 54 elite commercial rhizobial strains. Appl. Microbiol. Biotechnol. 2009, 83, 897–908. [Google Scholar] [CrossRef]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny: The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Faria, S.M.; Lima, H.C. Additional studies of the nodulation status of Legume species in Brazil. Plant Soil 1998, 200, 185–192. [Google Scholar] [CrossRef]
- Sprent, J.I.; Ardley, J.; James, E.K. Biogeography of nodulated legumes and their Nitrogen-fixing symbionts. New Phytol. 2017, 215, 40–56. [Google Scholar] [CrossRef]
- Andrade, C.M.S.; Salman, A.K.D.; Oliveira, T.K. Guia Arbopasto: Manual de Identificação e Seleção de Espécies Arbóreas Para Sistemas Silvipastoris; EMBRAPA: Brasília, Brazil, 2012. [Google Scholar]
- Schroth, G.; Kolbe, D.; Pity, B.; Zech, W. Root system characteristics with agroforestry relevance of nine leguminous tree species and a spontaneous fallow in a Semi-Deciduous Rainforest Area of West Africa. For. Ecol. Manag. 1996, 84, 199–208. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras; Jardim Botânico Plantarum: Nova Odessa, Brazil, 2020; ISBN 9786587655000. [Google Scholar]
- Cagnin Pereira, T.; Cardoso, L.P.; Da Silva De Paiva, W.; Santos De Camargos, L.; Cristina Rodrigues-Lisoni, F.; Redondo Martins, A. Analysis of metabolic compounds and antitumorigenic effects of Albizia niopoides and Senegalia polyphylla leaves. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Duan, M.; Chang, S.X. Nitrogen fertilization improves the growth of Lodgepole Pine and White Spruce seedlings under low salt stress through enhancing photosynthesis and plant nutrition. For. Ecol. Manag. 2017, 404, 197–204. [Google Scholar] [CrossRef]
- Soares, C.B.; Freitas, E.C.S.D.; Paiva, H.N.D.; Neves, J.C.L. Nitrogen sources and doses on growth and quality of seedlings of Cassia grandis and Peltophorum dubium 1. Rev. Árvore 2017, 41, e410214. [Google Scholar] [CrossRef][Green Version]
- Tosta, M.D.S.; Almeida, J.P.N.D.; Góes, G.B.D.; Freire, P.D.A.; Mendonça, V. Nitrogen fertilization in the production of seedlings of Talisia esculenta (A. St. Hil) Radlk. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 443–447. [Google Scholar] [CrossRef]
- Konzock, O.; Zaghen, S.; Fu, J.; Kerkhoven, E.J. Urea is a drop-in nitrogen source alternative to ammonium sulphate in Yarrowia lipolytica. iScience 2022, 25, 105703. [Google Scholar] [CrossRef]
- Góes, G.S.; Gross, E.; Brito-Rocha, E.; Mielke, M.S. Efeitos da inoculação com bactérias diazotróficas e da adubação nitrogenada no crescimento e na qualidade de mudas de Inga laurina (SW.) Willd. (Fabaceae)1. Rev. Árvore 2015, 39, 1031–1038. [Google Scholar] [CrossRef]
- Franco, A.A.; Faria, S.M. The contribution of N2-Fixing Tree Legumes to land reclamation and sustainability in the Tropics. Soil Biol. Biochem. 1997, 29, 897–903. [Google Scholar] [CrossRef]
- Fred, E.B.; Waksman, S.A. Laboratory Manual of General Microbiology; McGraw-Hill: New York, NY, USA, 1928. [Google Scholar]
- Urenha, L.C.; Pradella, J.G.C.; Oliveira, M.S.; Bonomi, A. Manual de Métodos Empregados em Estudos de Microbiologia Agrícola; EMBRAPA: Brasília, Brazil, 1994. [Google Scholar]
- Silva, R.B.G.D.; Gabira, M.M.; Prado, D.Z.D.; Uesugi, G.; Simões, D.; Silva, M.R.D. Influence of mean leaf angles and irrigation volumes on water capture, leaching, and growth of tropical tree seedlings. Forests 2020, 11, 1198. [Google Scholar] [CrossRef]
- Sorreano, M.C.M.; Rodrigues, R.R.; Boaretto, A.E. Guia de Nutrição para Espécies Florestais Nativas; Oficina de Textos: São Paulo, Brazil, 2012; ISBN 978-85-7975-049-6. [Google Scholar]
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Quality Appraisal of White Spruce and White Pine Seedling Stock in Nurseries. For. Chron. 1960, 36, 10–13. [Google Scholar] [CrossRef]
- Malavolta, E.; Vitti, G.C. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Ratkowsky, D.A. Handbook of Nonlinear Regression Models; Statistics, Textbooks and Monographs; M. Dekker: New York, NY, USA, 1990; ISBN 978-0-8247-8189-7. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. Gen. 1972, 135, 370. [Google Scholar] [CrossRef]
- Diggle, P. (Ed.) Analysis of Longitudinal Data, 2nd ed.; Oxford Statistical Science Series; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-967675-0. [Google Scholar]
- Westfall, P.H.; Tobias, R.D.; Westfall, P.H. Multiple Comparisons and Multiple Tests: Using the SAS System Workbook; Workbook Edition; 3rd printing; SAS Institute: Cary, NC, USA, 2008; ISBN 978-1-58025-759-6. [Google Scholar]
- Ndhlovu, K.; Bopape, F.L.; Diale, M.O.; Mpai, T.; Morey, L.; Mtsweni, N.P.; Gerrano, A.S.; Vuuren, A.V.; Babalola, O.O.; Hassen, A.I. Characterization of nodulation-compatible strains of native soil rhizobia from the rhizosphere of Soya Bean (Glycine max L.) fields in South Africa. Nitrogen 2024, 5, 1107–1123. [Google Scholar] [CrossRef]
- Parker, M.A. The spread of Bradyrhizobium lineages across host legume clades: From Abarema to Zygia. Microb. Ecol. 2015, 69, 630–640. [Google Scholar] [CrossRef]
- Ngwenya, Z.D.; Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Phylogenetic relationships among Bradyrhizobium species nodulating Groundnut (Arachis hypogea L.), Jack Bean (Canavalia ensiformis L.) and Soybean (Glycine max Merr.) in Eswatini. Sci. Rep. 2022, 12, 10629. [Google Scholar] [CrossRef] [PubMed]
- Puozaa, D.K.; Jaiswal, S.K.; Dakora, F.D. Phylogeny and distribution of Bradyrhizobium symbionts nodulating Cowpea (Vigna unguiculata L. Walp) and their association with the physicochemical properties of acidic African soils. Syst. Appl. Microbiol. 2019, 42, 403–414. [Google Scholar] [CrossRef]
- Hungria, M.; Menna, P.; Delamuta, J.R.M. Bradyrhizobium, the ancestor of all rhizobia: Phylogeny of housekeeping and Nitrogen-Fixation genes. In Biological Nitrogen Fixation; De Bruijn, F.J., Ed.; Wiley: Warsaw, Poland, 2015; pp. 191–202. ISBN 978-1-118-63704-3. [Google Scholar]
- Bournaud, C.; Faria, S.M.; Santos, J.M.F.; Tisseyre, P.; Silva, M.; Chaintreuil, C.; Gross, E.; James, E.K.; Prin, Y.; Moulin, L. Burkholderia species are the most common and preferred nodulating symbionts of the Piptadenia group (Tribe Mimoseae). PLoS ONE 2013, 8, e63478. [Google Scholar] [CrossRef]
- Mwenda, G.M.; Hill, Y.J.; O’Hara, G.W.; Reeve, W.G.; Howieson, J.G.; Terpolilli, J.J. Competition in the Phaseolus vulgaris-Rhizobium symbiosis and the role of resident soil rhizobia in determining the outcomes of inoculation. Plant Soil 2023, 487, 61–77. [Google Scholar] [CrossRef]
- Mahdhi, M.; Tounekti, T.; Khemira, H. Phenotypic and genotypic characterization of microsymbionts of Acacia species plants grown in South-Western Saudi Arabia. J. Soil Sci. Plant Nutr. 2019, 19, 631–638. [Google Scholar] [CrossRef]
- Zangaro, W.; Nisizaki, S.M.A.; Domingos, J.C.B.; Nakano, E.M. Micorriza Arbuscular em espécies arbóreas nativas da Bacia do Rio Tibagi, Paraná. CERNE 2002, 8, 77–87. [Google Scholar]
- Wang, X.; Feng, H.; Wang, Y.; Wang, M.; Xie, X.; Chang, H.; Wang, L.; Qu, J.; Sun, K.; He, W.; et al. Mycorrhizal symbiosis modulates the rhizosphere microbiota to promote Rhizobia–Legume symbiosis. Mol. Plant 2021, 14, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Júnior, J.Q.D.O.; Jesus, E.D.C.; Lisboa, F.J.; Berbara, R.L.L.; Faria, S.M.D. Nitrogen-Fixing Bacteria and Arbuscular Mycorrhizal Fungi in Piptadenia gonoacantha (Mart.) Macbr. Braz. J. Microbiol. 2017, 48, 95–100. [Google Scholar] [CrossRef]
- Valentine, A.J.; Kleinert, A.; Benedito, V.A. Adaptive strategies for nitrogen metabolism in phosphate deficient legume nodules. Plant Sci. 2017, 256, 46–52. [Google Scholar] [CrossRef]
- Lesueur, D.; Founoune, H.; Lebonvallet, S.; Sarr, A. Effect of rhizobial inoculation on growth of Calliandra tree species under nursery conditions. New For. 2010, 39, 129–137. [Google Scholar] [CrossRef]
- Porto, D.S.; Farias, E.D.N.C.; Chaves, J.D.S.; Souza, B.F.; Medeiros, R.D.D.; Zilli, J.É.; Silva, K.D. Symbiotic effectiveness of Bradyrhizobium ingae in promoting growth of Inga edulis Mart. seedlings. Rev. Bras. Ciênc. Solo 2017, 41, e0160222. [Google Scholar] [CrossRef]
- Diabate, M.; Munive, A.; Faria, S.M.; Ba, A.; Dreyfus, B.; Galiana, A. Occurrence of nodulation in unexplored leguminous trees native to the West African Tropical Rainforest and inoculation response of native species useful in reforestation. New Phytol. 2005, 166, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, W.; Wei, T.; Huang, R.; Zeng, Z. Patterns and mechanisms of legume responses to nitrogen enrichment: A global Meta-Analysis. Plants 2024, 13, 3244. [Google Scholar] [CrossRef]
- Mortier, V.; Holsters, M.; Goormachtig, S. Never too many? How Legumes control nodule numbers. Plant Cell Environ. 2012, 35, 245–258. [Google Scholar] [CrossRef]
- Li, Y.; Pei, Y.; Shen, Y.; Zhang, R.; Kang, M.; Ma, Y.; Li, D.; Chen, Y. Progress in the self-regulation system in legume nodule development-AON (Autoregulation of Nodulation). Int. J. Mol. Sci. 2022, 23, 6676. [Google Scholar] [CrossRef]
- Okuma, N.; Kawaguchi, M. Systemic optimization of legume nodulation: A shoot-derived regulator, miR2111. Front. Plant Sci. 2021, 12, 682486. [Google Scholar] [CrossRef]
- Dan, T.H.; Brix, H. Growth responses of the perennial legume Sesbania sesban to NH4 and NO3 nutrition and effects on root nodulation. Aquat. Bot. 2009, 91, 238–244. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Shabir, P.A.; Hakeem, K.R. Advances in Plant Nitrogen Metabolism, 1st ed.; CRC Press: New York, NY, USA, 2022; ISBN 978-1-00-324836-1. [Google Scholar]
- Grossnickle, S.C.; MacDonald, J.E. Why seedlings grow: Influence of plant attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Ni, M.; Liu, Y.; Chu, C.; Xu, H.; Fang, S. Fast seedling root growth leads to competitive superiority of invasive plants. Biol. Invasions 2018, 20, 1821–1832. [Google Scholar] [CrossRef]
- Deaker, R. Legume seed inoculation technology? A Review. Soil Biol. Biochem. 2004, 36, 1275–1288. [Google Scholar] [CrossRef]


| Clay | Silt | Sand | pH (CaCl2) | Organic matter | P (resin) |
| ------------ % ------------ | ----- g dm−3 ----- | --- mg dm−3 --- | |||
| 16 | 4 | 80 | 3.9 | 5 | 4 |
| H + Al | K | Ca | Mg | SB | CEC |
| ------------------------------------ mmolc dm−3 ------------------------------------ | |||||
| 31 | 0.9 | 4.0 | 1.0 | 5 | 36 |
| Variables | Inoculated | Non-Inoculated | ||
|---|---|---|---|---|
| Height | 8.83 (8.20–9.47) | 7.93 (7.42–8.44) | ns | |
| 0.021 (0.014–0.028) | 0.014 (0.010–0.0175) | ns | ||
| 605 (604–606) | 1093 (803–1382) | * | ||
| 13.56 cm | 13.49 cm | |||
| Adjusted R2 | 40% | 52% | ||
| p-value | 1.0 | 1.0 | ||
| Collar diameter | 2.75 (2.65–2.85) | 2.435 (2.34–2.53) | * | |
| 0.0033 (0.0023–0.0043) | 0.0040 (0.0032–0.0047) | ns | ||
| 695 (532–861) | 872 (711–1033) | ns | ||
| 3.59 mm | 3.70 mm | |||
| Adjusted R2 | 45% | 66% | ||
| p-value | 0.2318 | 0.0608 | ||
| Shoot dry mass | = max. | 5.92 (5.82–6.02) | ||
| = α = mean | 0.0070 (0.0012–0.0013) | |||
| 1078 (374–1781) | ||||
| 560.7 mg | 512.5 mg | |||
| Adjusted R2 | 9.14% | |||
| p-value | 1.000 | |||
| Root dry mass | 2050 (1763–2336) | 1842 (1573–2110) | ns | |
| 19.53 (10.1–30.0) | 14 (9.6–18.4) | ns | ||
| 276 (182–370) | 409 (314–504) | ns | ||
| 4033 mg | 3945 mg | |||
| Adjusted R2 | 30.43% | 41.34% | ||
| Total dry mass | 2608 (2288–2928) | 2282 (2058–2505) | ns | |
| 18.84 (9.5–28.2) | 13.80 (9.0–18.6) | ns | ||
| 285 (182–387) | 422 (320–524) | ns | ||
| 4587 mg | 4425 mg | |||
| Adjusted R2 | 27.44% | 40.05% | ||
| p-value | 0.647 | 0.999 |
| Shoot N (%) | Root N (%) | ||
|---|---|---|---|
| Inoculation | Both Inoculation Treatments (No Interaction) | ||
| N Rates | − | + | |
| 0 | 1.78 E b (0.02) | 3.18 C a (0.016) | 1.83 C (0.167) |
| 250 | 2.15 D b (0.073) | 3.05 C a (0.056) | 1.43 D (0.043) |
| 500 | 2.84 C b (0.56) | 3.09 C a (0.070) | 1.84 C (0.021) |
| 1000 | 3.67 B a (0.074) | 3.78 B a (0.033) | 2.71 B (0.104) |
| 2000 | 4.49 A a (0.048) | 4.43 A a (0.054) | 4.23 A (0.105) |
| N Fertilization | Inoculation | Interaction | ||||
|---|---|---|---|---|---|---|
| Chi2 | Pr > Chi2 | Chi2 | Pr > Chi2 | Chi2 | Pr > Chi2 | |
| HtI | 11.14 | <0.025 * | 6.85 | 0.009 ** | 9.63 | 0.0471 * |
| CDI | 14.89 | 0.0049 ** | 14.94 | 0.0001 ** | 5.91 | 0.2061 |
| LNI | 17.98 | <0.0012 ** | 14.45 | 0.0001 ** | 9.89 | 0.0422 * |
| ERDM | 31.40 | <0.0001 ** | 50.82 | <0.0001 ** | 21.94 | 0.0003 ** |
| HI | LNI | REM | ||||
|---|---|---|---|---|---|---|
| N | − | + | − | + | − | + |
| 0 | 3.46 A a (0.78) | 6.67 A a (0.70) | 4.33 ab A (1.14) | 5.00 A a (1.09) | 0.195 B b (0.03) | 1.383 A a (0.29) |
| 250 | 3.90 A a (1.07) | 6.71 A a (2.47) | 1.50 B b (0.56) | 5.67 A a (1.11) | 0.708 A a (0.16) | 1.298 A a (0.15) |
| 500 | 9.25 A a (2.97) | 5.80 A a (1.62) | 4.67 AB a (1.56) | 7.00 A a (1.71) | 0.805 A a (0.13) | 1.427 A a (0.15) |
| 1000 | 4.43 A a (1.37) | 11.20 a A (3.48) | 4.17 AB a (1.56) | 5.83 A a (1.40) | 1.040 A a (0.19) | 2.073 A a (0.32) |
| 2000 | 8.68 A a (1.72) | 11.67 a A (3.42) | 6.33 A a (1.71) | 7.00 A a (1.71) | 0.750 A b (0.14) | 1.580 A a (0.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.B.; Pieri, C.d.; Costa, L.J.S.d.; Luz, M.N.d.; Ganga, A.; Capra, G.F.; Passos, J.R.d.S.; Silva, M.R.d.; Guerrini, I.A. Inoculation with Bradyrhizobium elkanii Reduces Nitrogen Fertilization Requirements for Pseudalbizzia niopoides, a Multipurpose Neotropical Legume Tree. Nitrogen 2025, 6, 26. https://doi.org/10.3390/nitrogen6020026
Silva RB, Pieri Cd, Costa LJSd, Luz MNd, Ganga A, Capra GF, Passos JRdS, Silva MRd, Guerrini IA. Inoculation with Bradyrhizobium elkanii Reduces Nitrogen Fertilization Requirements for Pseudalbizzia niopoides, a Multipurpose Neotropical Legume Tree. Nitrogen. 2025; 6(2):26. https://doi.org/10.3390/nitrogen6020026
Chicago/Turabian StyleSilva, Rafael Barroca, Cristiane de Pieri, Leonardo José Silva da Costa, Mellina Nicácio da Luz, Antonio Ganga, Gian Franco Capra, José Raimundo de Souza Passos, Magali Ribeiro da Silva, and Iraê Amaral Guerrini. 2025. "Inoculation with Bradyrhizobium elkanii Reduces Nitrogen Fertilization Requirements for Pseudalbizzia niopoides, a Multipurpose Neotropical Legume Tree" Nitrogen 6, no. 2: 26. https://doi.org/10.3390/nitrogen6020026
APA StyleSilva, R. B., Pieri, C. d., Costa, L. J. S. d., Luz, M. N. d., Ganga, A., Capra, G. F., Passos, J. R. d. S., Silva, M. R. d., & Guerrini, I. A. (2025). Inoculation with Bradyrhizobium elkanii Reduces Nitrogen Fertilization Requirements for Pseudalbizzia niopoides, a Multipurpose Neotropical Legume Tree. Nitrogen, 6(2), 26. https://doi.org/10.3390/nitrogen6020026










