Activated Carbon Reduced Nitrate Loss from Agricultural Soil but Did Not Enhance Wheat Yields
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils and Amendments
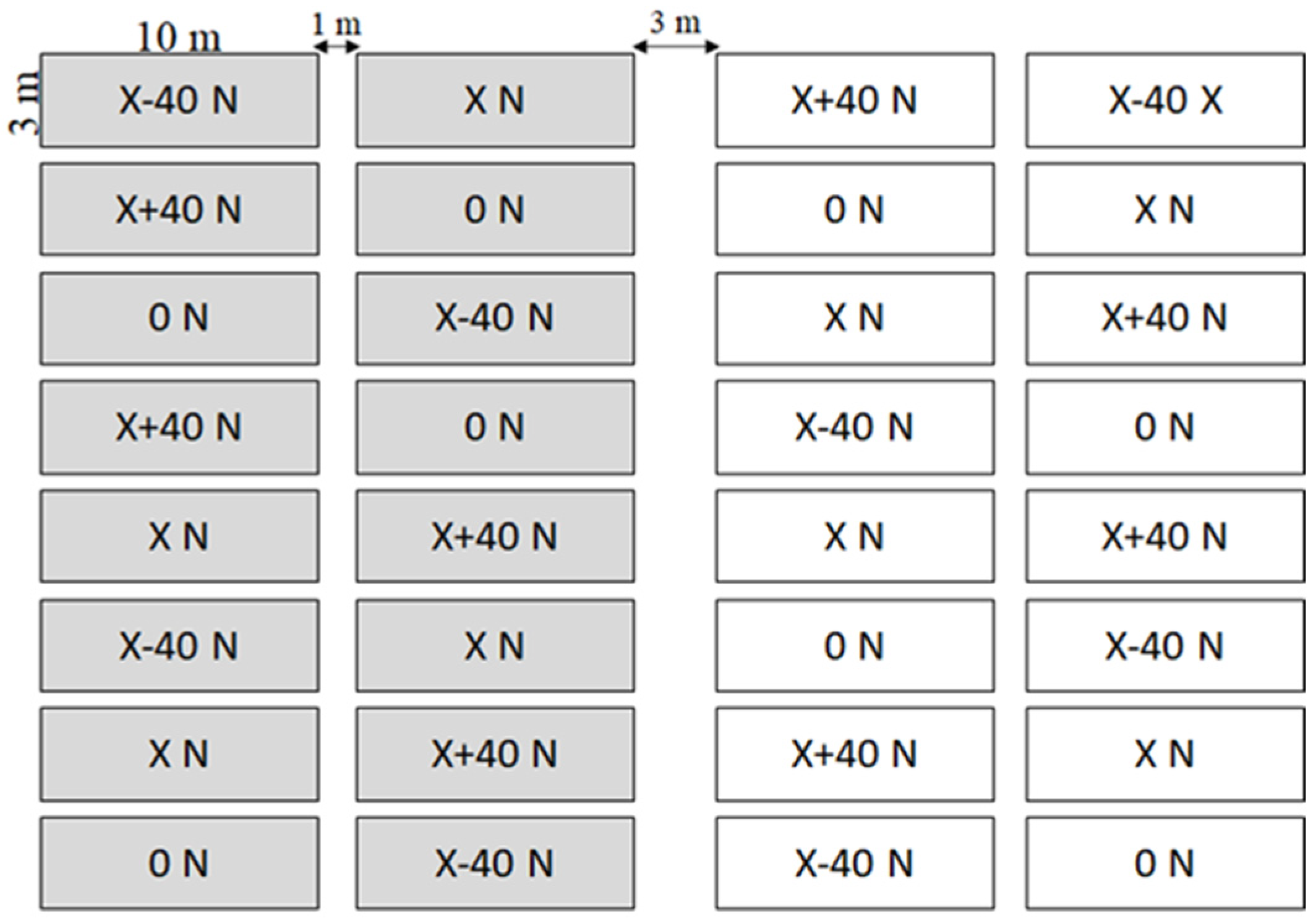
2.2. Microcosm Laboratory Experiment: Column Leaching Test
2.3. Pot Culture Test
2.4. Field Validation
2.5. Data Analysis
3. Results
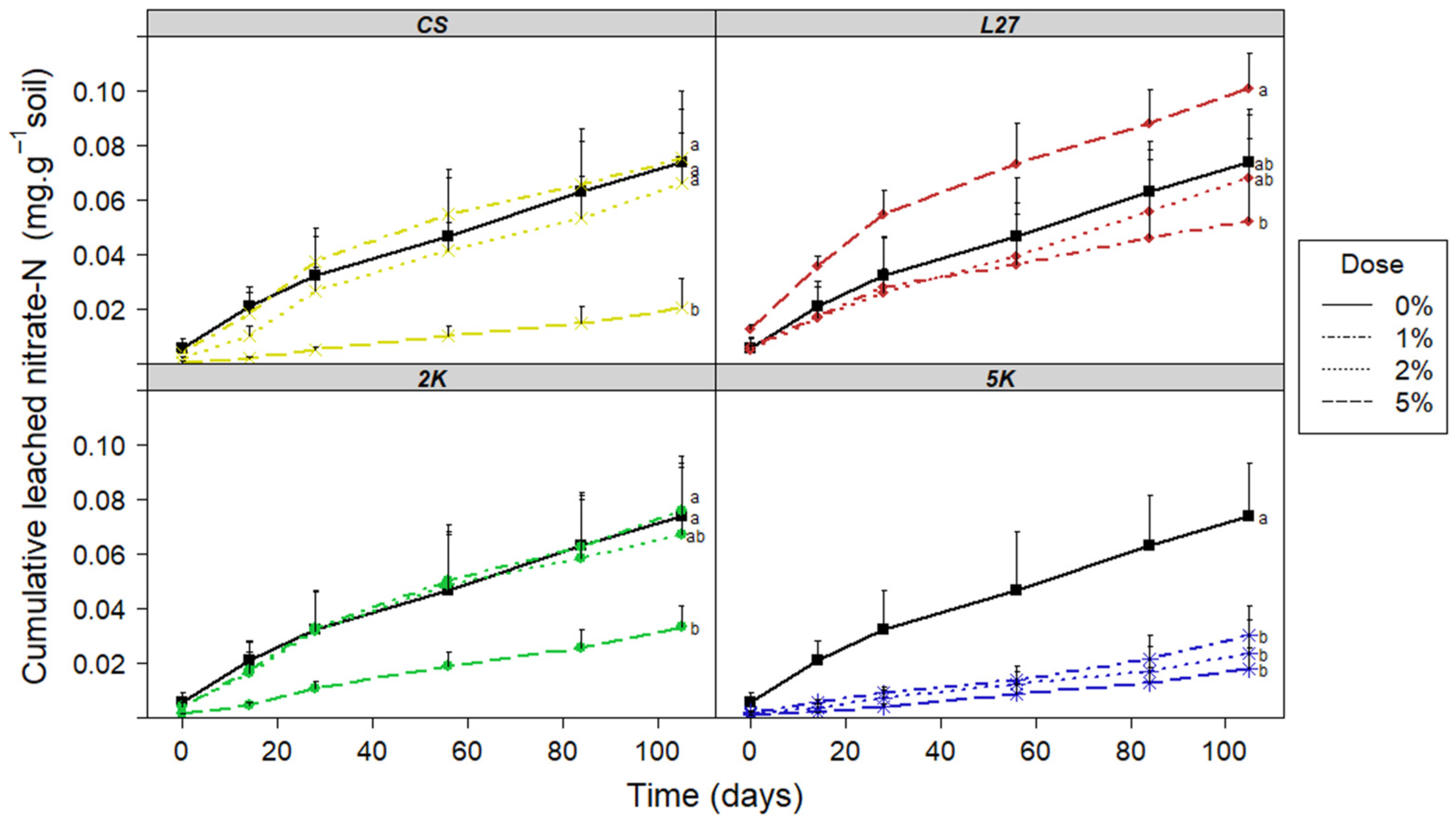
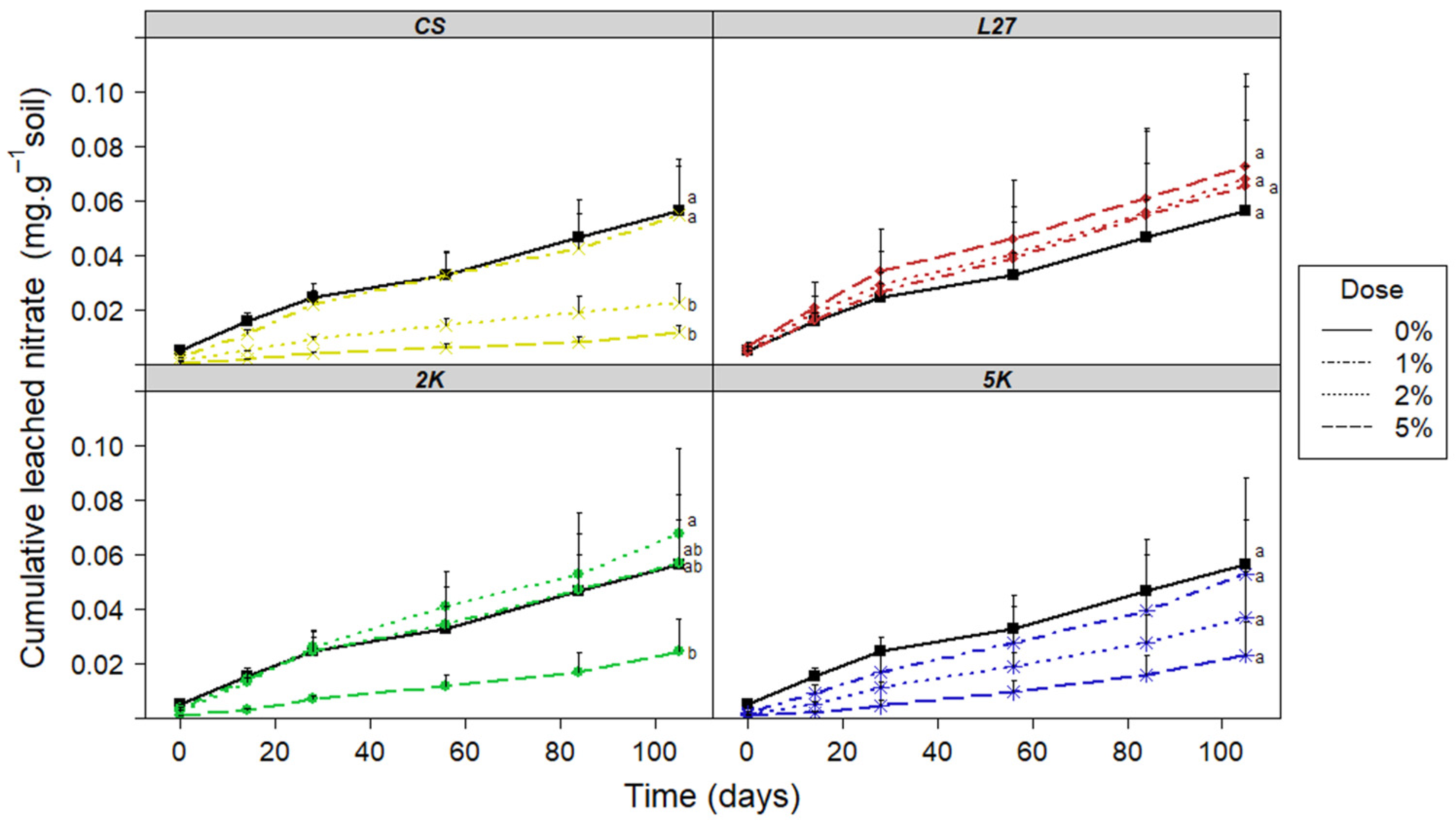
3.1. Leaching Column Test
3.1.1. Leachate Analysis
3.1.2. Soil Analysis
3.2. Pot Experiment
3.2.1. Leachate Analysis
3.2.2. Plant Analysis
3.2.3. Soil Analysis
3.3. Field Experiment
4. Discussion
4.1. The Application of Activated Carbon Stabilizes Carbon in the Soil, Potential for Carbon Storage
4.2. Activated Carbon and Plant Development Allows for a Reduction in the Leaching of Nitrate as Well as the Potential for a Reduction in Chemical Fertilizer Use
4.3. Activated Carbon Diminishes the Functional Diversity of the Soil, Producing a Negative Effect on Microbes
4.4. Plant Growth Is Not Enhanced by Activated Carbon Addition
4.5. The Effect of Activated Carbon Is Modulated by the Activated Carbon and Soil Type
4.6. Practical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OM | organic matter |
| OC | organic carbon |
| TOC | total organic carbon |
| AC | activated carbon |
| S | Saugy |
| T | Tournoisis |
| CEC | cation exchange capacity |
| AWCD | average well color development |
| PNP | p nitrophenol |
References
- Duchene, O.; Celette, F.; Ryan, M.R.; DeHaan, L.R.; Crews, T.E.; David, C. Integrating Multipurpose Perennial Grains Crops in Western European Farming Systems. Agric. Ecosyst. Environ. 2019, 284, 106591. [Google Scholar] [CrossRef]
- Le Gouis, J.; Oury, F.-X.; Charmet, G. How Changes in Climate and Agricultural Practices Influenced Wheat Production in Western Europe. J. Cereal Sci. 2020, 93, 102960. [Google Scholar] [CrossRef]
- Singh, B. Are Nitrogen Fertilizers Deleterious to Soil Health? Agronomy 2018, 8, 48. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of Biochar for the Removal of Nitrogen and Phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; G.K., S.; Vadivel, R.; T.K., D.; et al. Nitrogen Use Efficiency—A Key to Enhance Crop Productivity under a Changing Climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Khanna, R.; Gupta, S. Agrochemicals as a Potential Cause of Ground Water Pollution: A Review. Int. J. Chem. Stud. 2018, 6, 985–990. [Google Scholar]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the Environment: A Critical Review of Their Distribution, Sensing Techniques, Ecological Effects and Remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Ravi Kanth Reddy, P.; Obaisi, A.I.; Elghandour, M.M.M.Y.; Oyebamiji, K.J.; Salem, A.Z.M.; Morakinyo-Fasipe, O.T.; Cipriano-Salazar, M.; Camacho-Díaz, L.M. Sustainable Agriculture Options for Production, Greenhouse Gasses and Pollution Alleviation, and Nutrient Recycling in Emerging and Transitional Nations—An Overview. J. Clean. Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Cicekalan, B.; Cengiz, A.I.; Zhang, X.; Ozgun, H. Nutrient Recovery from Municipal Solid Waste Leachate in the Scope of Circular Economy: Recent Developments and Future Perspectives. J. Environ. Manag. 2023, 335, 117518. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A Complete Review on Biochar: Production, Property, Multifaceted Applications, Interaction Mechanism and Computational Approach. Fuel 2021, 292, 120243. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Wang, H.; Liu, J.; Zhai, L.; Liu, S.; Ren, T.; Liu, H. Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate. PLoS ONE 2014, 9, e113888. [Google Scholar] [CrossRef] [PubMed]
- Basalirwa, D.; Sudo, S.; Wacal, C.; Namirembe, C.; Sasagawa, D.; Yamamoto, S.; Masunaga, T.; Nishihara, E. Effect of Activated Carbon on Greenhouse Gas Emissions, Seed Yield, Soil Chemical Properties and Isoflavone Content of Soybean Genotypes with Varying Nodulation Capacities under Sandy Soil Conditions. Rhizosphere 2020, 14, 100202. [Google Scholar] [CrossRef]
- Hanafi, H.A.; Azeema, S.M.A.b. Removal of Nitrate and Nitrite Anions from Wastewater Using Activated Carbon Derived from Rice Straw. J. Environ. Anal. Toxicol. 2016, 6, 2161-0525. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahn, Y.-T.; Kumar, M.; Lee, W.; Min, B.; Kim, G.; Cho, D.-W.; Park, W.B.; Jeon, B.-H. Adsorption Studies for the Removal of Nitrate Using Modified Lignite Granular Activated Carbon. Sep. Sci. Technol. 2011, 46, 2575–2584. [Google Scholar] [CrossRef]
- Lebrun, M.; Renouard, S.; Morabito, D.; Bourgerie, S. Sorption Capacities of Various Activated Carbons towards Nitrates: Effects of Nitrate Concentration, PH, Time and Co-Existing Ions. Int. J. Environ. Sci. Technol. 2023, 20, 13033–13044. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic Biochar for Environmental Remediation: A Review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Li, N.; Nie, M.; Li, B.; Wu, J.; Zhao, J. Contrasting Effects of the Aboveground Litter of Native Phragmites Australis and Invasive Spartina Alterniflora on Nitrification and Denitrification. Sci. Total Environ. 2021, 764, 144283. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Van Poucke, R.; Tack, F.M.G.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Effect of Fertilization, Carbon-based Material, and Redmud Amendments on the Bacterial Activity and Diversity of a Metal(Loid) Contaminated Mining Soil. Land Degrad. Dev. 2021, 32, 2618–2628. [Google Scholar] [CrossRef]
- García-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-Term Effects of Municipal Solid Waste Compost Application on Soil Enzyme Activities and Microbial Biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Schnrer, J.; Rosswall, T. Fluorescein Diacetate Hydrolysis as a Measure of Total Microbial Activity in Soil and Litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Cordero, I.; Snell, H.; Bardgett, R.D. High Throughput Method for Measuring Urease Activity in Soil. Soil Biol. Biochem. 2019, 134, 72–77. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Li, M.; Wang, J.; Guo, D.; Yang, R.; Fu, H. Effect of Land Management Practices on the Concentration of Dissolved Organic Matter in Soil: A Meta-Analysis. Geoderma 2019, 344, 74–81. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Barnes, R.T.; Gallagher, M.E.; Gonnermann, H. Impacts of Biochar Concentration and Particle Size on Hydraulic Conductivity and DOC Leaching of Biochar–Sand Mixtures. J. Hydrol. 2016, 533, 461–472. [Google Scholar] [CrossRef]
- Daryabeigi Zand, A.; Grathwohl, P. Enhanced Immobilization of Polycyclic Aromatic Hydrocarbons in Contaminated Soil Using Forest Wood-Derived Biochar and Activated Carbon under Saturated Conditions, and the Importance of Biochar Particle Size. Pol. J. Environ. Stud. 2016, 25, 427–441. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. High Temperature-Produced Biochar Can Be Efficient in Nitrate Loss Prevention and Carbon Sequestration. Geoderma 2019, 338, 48–55. [Google Scholar] [CrossRef]
- Hale, S.E.; Elmquist, M.; Brändli, R.; Hartnik, T.; Jakob, L.; Henriksen, T.; Werner, D.; Cornelissen, G. Activated Carbon Amendment to Sequester PAHs in Contaminated Soil: A Lysimeter Field Trial. Chemosphere 2012, 87, 177–184. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Hamdan, R.; Cooper, W.T. Physicochemical Changes in Pyrogenic Organic Matter (Biochar) after 15 Months Field-Aging. Solid Earth 2014, 5, 693–704. [Google Scholar] [CrossRef]
- Sandhu, S.S.; Ussiri, D.A.N.; Kumar, S.; Chintala, R.; Papiernik, S.K.; Malo, D.D.; Schumacher, T.E. Analyzing the Impacts of Three Types of Biochar on Soil Carbon Fractions and Physiochemical Properties in a Corn-Soybean Rotation. Chemosphere 2017, 184, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ning, L.; Xun, M.; Feng, F.; Li, P.; Yue, S.; Song, J.; Zhang, W.; Yang, H. Biochar Can Increase Nitrogen Use Efficiency of Malus Hupehensis by Modulating Nitrate Reduction of Soil and Root. Appl. Soil Ecol. 2019, 135, 25–32. [Google Scholar] [CrossRef]
- Ghorbani, M.; Asadi, H.; Abrishamkesh, S. Effects of Rice Husk Biochar on Selected Soil Properties and Nitrate Leaching in Loamy Sand and Clay Soil. Int. Soil Water Conserv. Res. 2019, 7, 258–265. [Google Scholar] [CrossRef]
- Raave, H.; Keres, I.; Kauer, K.; Nõges, M.; Rebane, J.; Tampere, M.; Loit, E. The Impact of Activated Carbon on NO3−-N, NH4+-N, P and K Leaching in Relation to Fertilizer Use: Impact of Activated Carbon on NO3−, NH4+, P, K Leaching. Eur. J. Soil Sci. 2014, 65, 120–127. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, X.; Singh, B.P.; Mandal, S.; Guo, J.; Che, L.; Wang, H. Effects of Contrasting Biochars on the Leaching of Inorganic Nitrogen from Soil. J. Soils Sediments 2020, 20, 3017–3026. [Google Scholar] [CrossRef]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of Carbon and Microbial Activity in Salt-Induced Soil by Application of Peanut Shell Biochar during Short-Term Incubation Study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef]
- Ding, X.; Li, G.; Zhao, X.; Lin, Q.; Wang, X. Biochar Application Significantly Increases Soil Organic Carbon under Conservation Tillage: An 11-Year Field Experiment. Biochar 2023, 5, 28. [Google Scholar] [CrossRef]
- Mustafa, A.; Holatko, J.; Hammerschmiedt, T.; Kucerik, J.; Skarpa, P.; Kintl, A.; Racek, J.; Baltazar, T.; Malicek, O.; Brtnicky, M. Comparison of the Responses of Soil Enzymes, Microbial Respiration and Plant Growth Characteristics under the Application of Agricultural and Food Waste-Derived Biochars. Agronomy 2022, 12, 2428. [Google Scholar] [CrossRef]
- Ameur, D.; Zehetner, F.; Johnen, S.; Jöchlinger, L.; Pardeller, G.; Wimmer, B.; Rosner, F.; Faber, F.; Dersch, G.; Zechmeister-Boltenstern, S.; et al. Activated Biochar Alters Activities of Carbon and Nitrogen Acquiring Soil Enzymes. Pedobiologia 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of Pesticides on Microorganisms, Enzymatic Activity and Plant in Biochar-Amended Soil. Geoderma 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Song, D.; Xi, X.; Zheng, Q.; Liang, G.; Zhou, W.; Wang, X. Soil Nutrient and Microbial Activity Responses to Two Years after Maize Straw Biochar Application in a Calcareous Soil. Ecotoxicol. Environ. Saf. 2019, 180, 348–356. [Google Scholar] [CrossRef]
- Al Marzooqi, F.; Yousef, L.F. Biological Response of a Sandy Soil Treated with Biochar Derived from a Halophyte (Salicornia bigelovii). Appl. Soil Ecol. 2017, 114, 9–15. [Google Scholar] [CrossRef]
- Song, X.; Razavi, B.S.; Ludwig, B.; Zamanian, K.; Zang, H.; Kuzyakov, Y.; Dippold, M.A.; Gunina, A. Combined Biochar and Nitrogen Application Stimulates Enzyme Activity and Root Plasticity. Sci. Total Environ. 2020, 735, 139393. [Google Scholar] [CrossRef]
- Dominchin, M.F.; Verdenelli, R.A.; Berger, M.G.; Aoki, A.; Meriles, J.M. Impact of N-Fertilization and Peanut Shell Biochar on Soil Microbial Community Structure and Enzyme Activities in a Typic Haplustoll under Different Management Practices. Eur. J. Soil Biol. 2021, 104, 103298. [Google Scholar] [CrossRef]
- Asirifi, I.; Werner, S.; Heinze, S.; Saba, C.K.S.; Lawson, I.Y.D.; Marschner, B. Short-Term Effect of Biochar on Microbial Biomass, Respiration and Enzymatic Activities in Wastewater Irrigated Soils in Urban Agroecosystems of the West African Savannah. Agronomy 2021, 11, 271. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar Application to Low Fertility Soils: A Review of Current Status, and Future Prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar Affects Soil Organic Matter Cycling and Microbial Functions but Does Not Alter Microbial Community Structure in a Paddy Soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef]
- Schmidt, H.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Sánchez Monedero, M.A.; Cayuela, M.L. Biochar in Agriculture—A Systematic Review of 26 Global Meta-analyses. GCB Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Hansen, S.; Berland Frøseth, R.; Stenberg, M.; Stalenga, J.; Olesen, J.E.; Krauss, M.; Radzikowski, P.; Doltra, J.; Nadeem, S.; Torp, T.; et al. Reviews and Syntheses: Review of Causes and Sources of N2O Emissions and NO3 Leaching from Organic Arable Crop Rotations. Biogeosciences 2019, 16, 2795–2819. [Google Scholar] [CrossRef]
- Břendová, K.; Zemanová, V.; Pavlíková, D.; Tlustoš, P. Utilization of Biochar and Activated Carbon to Reduce Cd, Pb and Zn Phytoavailability and Phytotoxicity for Plants. J. Environ. Manag. 2016, 181, 637–645. [Google Scholar] [CrossRef]
- Brennan, A.; Jiménez, E.M.; Puschenreiter, M.; Alburquerque, J.A.; Switzer, C. Effects of Biochar Amendment on Root Traits and Contaminant Availability of Maize Plants in a Copper and Arsenic Impacted Soil. Plant Soil 2014, 379, 351–360. [Google Scholar] [CrossRef]
- Doan, T.T.; Henry-des-Tureaux, T.; Rumpel, C.; Janeau, J.-L.; Jouquet, P. Impact of Compost, Vermicompost and Biochar on Soil Fertility, Maize Yield and Soil Erosion in Northern Vietnam: A Three Year Mesocosm Experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Torabian, S.; Qin, R.; Noulas, C.; Lu, Y.; Gao, S. Biochar Effects on Yield of Cereal and Legume Crops Using Meta-Analysis. Sci. Total Environ. 2021, 775, 145869. [Google Scholar] [CrossRef]
- Nobile, C.; Lebrun, M.; Védère, C.; Honvault, N.; Aubertin, M.-L.; Faucon, M.-P.; Girardin, C.; Houot, S.; Kervroëdan, L.; Dulaurent, A.-M.; et al. Biochar and Compost Addition Increases Soil Organic Carbon Content and Substitutes P and K Fertilizer in Three French Cropping Systems. Agron. Sustain. Dev. 2022, 42, 119. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar Increases Soil Microbial Biomass with Changes in Extra- and Intracellular Enzyme Activities: A Global Meta-Analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]

| Saugy | Tournoisis | ||
|---|---|---|---|
| Soil texture | Clay limestone | Clay limestone | |
| Cation exchange capacity (meq/100 g) | 18.2 | 34.7 | |
| Total nitrogen (g.kg−1) | 2.16 | 2.27 | |
| Organic carbon (g.kg−1) | 24.2 | 20.9 | |
| Organic matter (g.kg−1) | 43.2 | 45.4 | |
| C/N | 11.2 | 9.2 | |
| pH (H2O) | 8.28 | 8.33 | |
| Olsen [P] (mg.kg−1) | 55 | 43 | |
| Exchangeable cation concentrations | K (mg.kg−1) | 416 | 437 |
| Ca (mg.kg−1) | 8476 | 11,129 | |
| Mg (mg.kg−1) | 79 | 211 | |
| Na (mg.kg−1) | 6.68 | 16.3 | |
| Total element concentrations | Cu (mg.kg−1) | 2.6 | 2 |
| Zn (mg.kg−1) | 3 | 1.4 | |
| Mn (mg.kg−1) | 25.2 | 16.9 | |
| Fe (mg.kg−1) | 7.8 | 9.3 | |
| Activated Carbon | pH | EC (µS.cm−1) | Eh (mV) | SSA (m2.g−1) * | TPV (cm3.g−1) * | MPD (nm) | C (%) | H (%) | N (%) |
|---|---|---|---|---|---|---|---|---|---|
| L27 | 1.9 ± 0.1 | 2680 ± 2 | 525 ± 1 | 1500 | 2.78 | 15 | 89 ± 5 | 2.10 ± 0.09 | 1.54 ± 0.16 |
| 5K | 10.5 ± 0.0 | 122 ± 18 | 51 ± 7 | 1060 | 1.77 | 15 | 85 ± 1 | 0.04 ± 0.03 | 2.85 ± 0.46 |
| 2K | 9.5 ± 0.0 | 5 ± 0 | 200 ± 4 | 1050 | 0.87 | 9 | 82 ± 2 | 0.36 ± 0.01 | 1.91 ± 0.79 |
| CS | 10.7 ± 0.0 | 349 ± 4 | 157 ± 2 | 1100 | 0.67 | 7 | 86 ± 4 | 0.59 ± 0.13 | 0.13 ± 0.02 |
| Enzyme | Substrate | Buffer | Absorbance Wavelength | Reference |
|---|---|---|---|---|
| β-glucosidase | 4-nitrophenyl-β-D-glucopyranoside (10 mM) | Citrate phosphate buffer (0.15 M, pH 4–5) | 410 nm | [20,21] |
| Alkaline phosphatase | 4-nitrophenyl phosphate disodium salt hexahydrate (5 mM) | Tris-HCl (0.1 M, pH 8) | 410 nm | [21,22] |
| Acid phosphatase | 4-nitrophenyl phosphate disodium salt hexahydrate (5 mM) | Sodium acetate (0.1 M, pH 5) | 410 nm | [21,22] |
| Hydrolysis | Fluorescein diacetate (50 mM) | Potassium phosphate (60 mM, pH 7.6) | 490 nm | [21,23] |
| Urease | Urea | Sodium acetate (50 mM, pH 5) | 650 nm | [24] |
| β Glucosidase (nmol Glc.g−1 soil.min−1) | Alkaline Phosphatase (nmol PNP.g−1 soil.min−1) | Acid Phosphatase (nmol PNP.g−1 soil.min−1) | Hydrolysis (nmol FDA.g−1 soil.min−1) | Urease (nmol NH4+.g−1 soil.min−1) | Nitrification Potential (mg NO2−.kg−1 soil.h−1) | |
|---|---|---|---|---|---|---|
| S | 1.46 ± 0.20 a | 0.04 ± 0.01 a | 0.13 ± 0.01 a | 11.24 ± 1.43 a | 1.01 ± 0.13 a | 0.88 ± 0.08 a |
| S+1%5K | 0.67 ± 0.30 b | 0.04 ± 0.00 a | 0.12 ± 0.02 a | 11.60 ± 1.15 a | 0.78 ± 0.09 a | 0.89 ± 0.22 a |
| S+2%5K | 0.58 ± 0.07 b | 0.03 ± 0.00 a | 0.11 ± 0.00 a | 8.84 ± 1.31 a | 0.96 ± 0.11 a | 0.88 ± 0.17 a |
| S+5%5K | 0.36 ± 0.10 b | 0.03 ± 0.00 a | 0.11 ± 0.02 a | 3.95 ± 0.97 b | 0.76 ± 0.04 a | 0.81 ± 0.20 a |
| T | 1.81 ± 0.40 a | 0.04 ± 0.00 a | 0.15 ± 0.01 a | 15.59 ± 0.78 a | 0.75 ± 0.10 ab | 1.63 ± 0.12 b |
| T+1%5K | 0.06 ± 0.06 c | 0.03 ± 0.00 a | 0.14 ± 0.01 a | 8.69 ± 0.36 b | 0.66 ± 0.08 b | 2.00 ± 0.13 ab |
| T+2%5K | 0.36 ± 0.15 bc | 0.04 ± 0.01 a | 0.14 ± 0.01 a | 4.41 ± 0.73 c | 1.24 ± 0.17 a | 2.38 ± 0.24 a |
| T+5%5K | 0.64 ± 0.08 ab | 0.04 ± 0.01 a | 0.13 ± 0.00 a | 3.11 ± 1.31 c | 0.98 ± 0.14 ab | 2.17 ± 0.17 ab |
| Organic Carbon (mg.L−1) | NO3−N (mg.L−1) | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T48 | T191 | T0 | T48 | T191 | ||
| S | No | 19.0 ± 0.9 a | 5.8 ± 0.4 a | 2.8 ± 0.5 b | 19.15 ± 6.87 a | 33.38 ± 2.31 ab | 52.08 ± 13.62 ab |
| Yes | / | 6.1 ± 0.2 a | 3.0 ± 0.6 b | / | 1.26 ± 0.63 d | 0.11 ± 0.01 e | |
| S+F | No | / | 7.0 ± 1.0 a | 4.3 ± 0.4 a | / | 43.83 ± 8.96 a | 93.74 ± 9.94 a |
| Yes | / | 6.7 ± 0.4 a | 2.3 ± 0.2 bc | / | 8.75 ± 3.40 cd | 0.11 ± 0.02 e | |
| S+AC0.5+F | No | 4.4 ± 0.4 b | 1.8 ± 0.2 b | 1.2 ± 0.1 c | 15.26 ± 3.83 a | 20.23 ± 2.70 bc | 64.26 ± 7.00 ab |
| Yes | / | 2.0 ± 0.2 b | 1.3 ± 0.1 c | / | 8.79 ± 2.87 d | 0.23 ± 0.08 cde | |
| S+AC1 | No | 3.9 ± 0.4 b | 1.9 ± 0.1 b | 1.4 ± 0.1 c | 14.16 ± 3.51 a | 10.94 ± 2.32 cd | 6.85 ± 3.16 bcd |
| Yes | / | 1.9 ± 0.1 b | 1.3 ± 0.1 c | / | 3.49 ± 1.14 d | 0.12 ± 0.03 de | |
| S+AC1+F | No | / | 2.1 ± 0.1 b | 1.2 ± 0.1 c | / | 22.07 ± 3.49 ab | 20.20 ± 3.21 abc |
| Yes | / | 1.9 ± 0.1 b | 1.2 ± 0.1 c | / | 7.82 ± 0.95 cd | 0.12 ± 0.03 e | |
| Aerial Biomass (g) | Root Biomass (g) | 1000-Grain Weight (g) | Carbon Content (%) | Nitrogen Content (%) | |
|---|---|---|---|---|---|
| S | 18.6 ± 0.45 b | 2.0 ± 0.4 b | 30.5 ± 2.1 b | 40.5 ± 0.3 b | 0.74 ± 0.10 a |
| S+F | 33.2 ± 1.5 a | 5.0 ± 1.3 a | 28.5 ± 3.8 b | 42.1 ± 0.4 a | 0.71 ± 0.08 a |
| S+AC0.5+F | 33.7 ± 1.3 a | 3.7 ± 0.8 ab | 40.7 ± 3.3 ab | 42.6 ± 0.8 a | 0.78 ± 0.08 a |
| S+AC1 | 19.7 ± 0.2 b | 2.1 ± 0.4 ab | 50.8 ± 5.3 a | 38.7 ± 0.5 c | 0.91 ± 0.06 a |
| S+AC1+F | 31.4 ± 1.7 a | 3.6 ± 0.7 ab | 39.7 ± 6.4 ab | 42.6 ± 0.2 a | 0.71 ± 0.05 a |
| T | 18.5 ± 0.8 b | 0.7 ± 0.2 a | 32.3 ± 5.9 a | 39.7 ± 0.3 b | 1.00 ± 0.08 a |
| T+F | 29.4 ± 1.6 a | 0.8 ± 0.2 a | 33.5 ± 7.3 a | 42.2 ± 0.1 a | 0.93 ± 0.04 a |
| T+AC0.5+F | 26.4 ± 2.5 a | 1.0 ± 0.3 a | 46.4 ± 7.9 a | 41.2 ± 0.3 a | 1.23 ± 0.15 a |
| T+AC1 | 15.7 ± 1.1 b | 0.5 ± 0.2 a | 28.1 ± 3.4 a | 41.1 ± 0.3 ab | 0.85 ± 0.04 a |
| T+AC1+F | 25.1 ± 1.8 a | 0.6 ± 0.2 a | 33.8 ± 4.0 a | 41.3 ± 0.9 a | 0.89 ± 0.03 a |
| Organic Carbon (mg.L−1) | NO3−N (mg.L−1) | ||||||
|---|---|---|---|---|---|---|---|
| T0 | T48 | T191 | T0 | T48 | T191 | ||
| T | No | 30.6 ± 2.8 a | 10.6 ± 0.6 a | 5.2 ± 1.7 ab | 5.82 ± 1.98 a | 37.03 ± 7.80 a | 61.12 ± 15.25 a |
| Yes | / | 11.1 ± 0.9 a | 2.7 ± 0.4 abc | / | 1.57 ± 0.62 e | 8.52 ± 2.63 b | |
| T+F | No | / | 9.5 ± 0.3 a | 5.8 ± 1.6 a | / | 41.76 ± 5.40 a | 98.31 ± 19.91 a |
| Yes | / | 10.5 ± 0.6 a | 3.9 ± 2.1 abc | / | 13.34 ± 1.72 bcd | 5.12 ± 2.18 b | |
| T+AC0.5+F | No | 11.5 ± 0.9 b | 2.6 ± 0.2 b | 3.2 ± 0.6 abc | 1.37 ± 0.10 b | 20.59 ± 4.17 abcd | 84.09 ± 11.28 a |
| Yes | / | 2.9 ± 0.2 b | 1.3 ± 0.1 c | / | 4.05 ± 0.88 de | 8.62 ± 4.26 b | |
| T+AC1 | No | 12.3 ± 1.3 b | 3.5 ± 0.3 b | 4.0± 1.1 abc | 1.27 ± 0.31 b | 18.60 ± 4.92 abc | 65.77 ± 16.12 a |
| Yes | / | 2.8 ± 0.2 b | 1.4 ± 0.1 c | / | 0.37 ± 0.13 e | 2.03 ± 0.82 b | |
| T+AC1+F | No | / | 3.1 ± 0.2 b | 3.6 ± 0.4 abc | / | 33.83 ± 5.28 ab | 108.06 ± 19.99 a |
| Yes | / | 3.0 ± 0.1 b | 2.9 ± 1.4 bc | / | 4.06 ± 1.39 cde | 7.67 ± 4.00 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebrun, M.; Bourgerie, S. Activated Carbon Reduced Nitrate Loss from Agricultural Soil but Did Not Enhance Wheat Yields. Nitrogen 2025, 6, 30. https://doi.org/10.3390/nitrogen6020030
Lebrun M, Bourgerie S. Activated Carbon Reduced Nitrate Loss from Agricultural Soil but Did Not Enhance Wheat Yields. Nitrogen. 2025; 6(2):30. https://doi.org/10.3390/nitrogen6020030
Chicago/Turabian StyleLebrun, Manhattan, and Sylvain Bourgerie. 2025. "Activated Carbon Reduced Nitrate Loss from Agricultural Soil but Did Not Enhance Wheat Yields" Nitrogen 6, no. 2: 30. https://doi.org/10.3390/nitrogen6020030
APA StyleLebrun, M., & Bourgerie, S. (2025). Activated Carbon Reduced Nitrate Loss from Agricultural Soil but Did Not Enhance Wheat Yields. Nitrogen, 6(2), 30. https://doi.org/10.3390/nitrogen6020030







