Direct Catalyst Conversion Sensor in Form of a Single Self-Heated Mixed-Potential Device †
Abstract
:1. Introduction
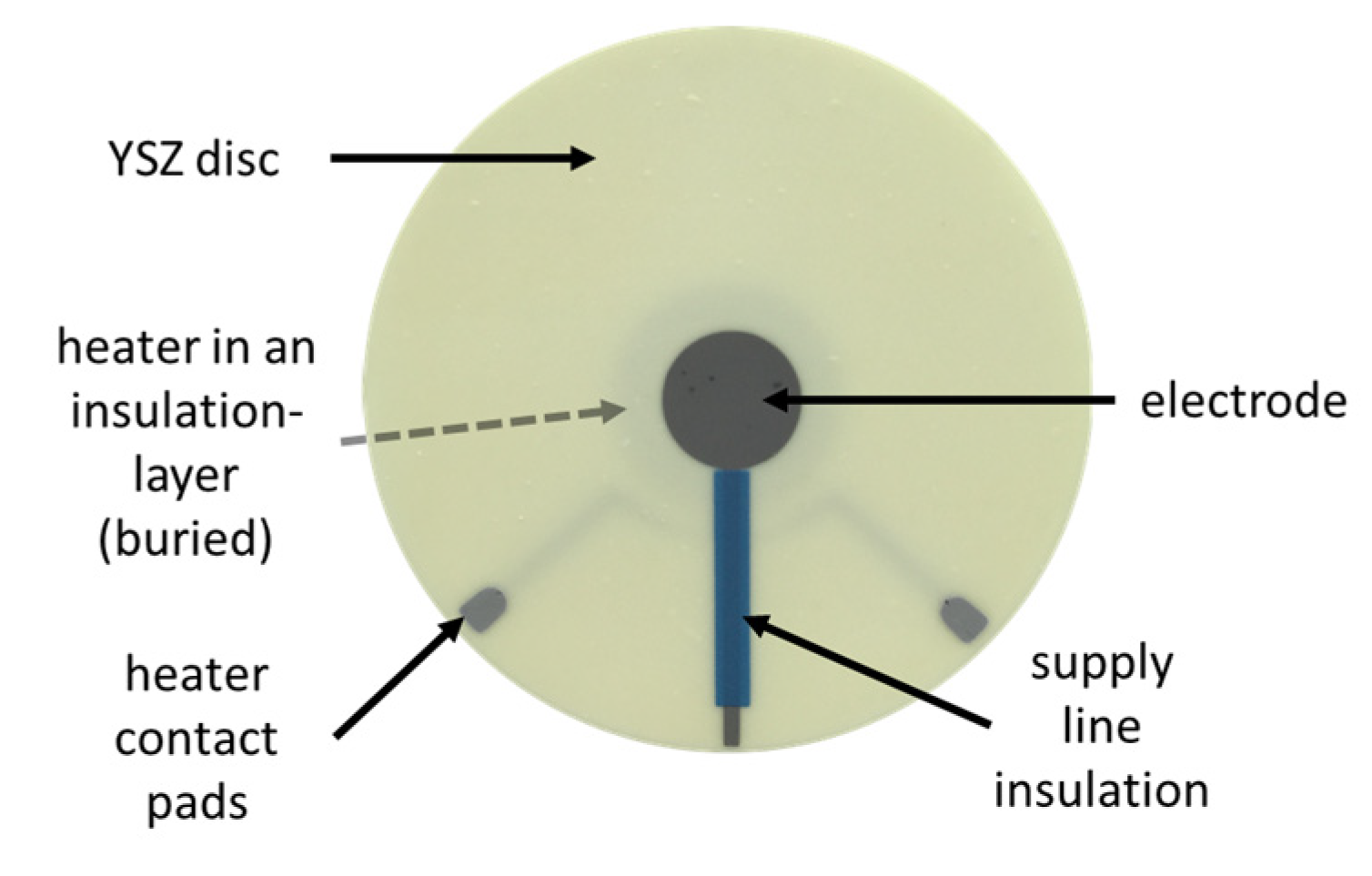
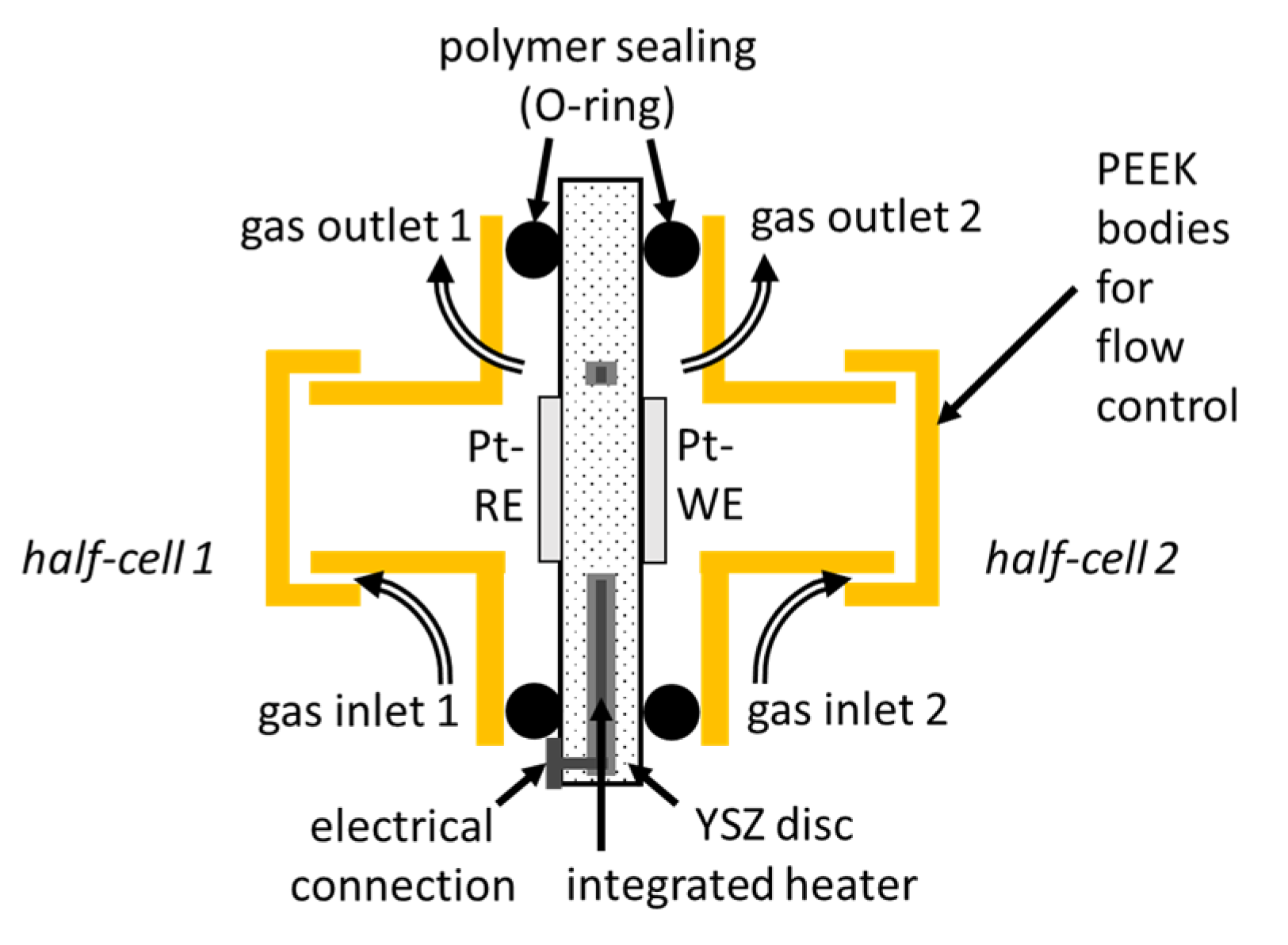
2. Sensing Principle
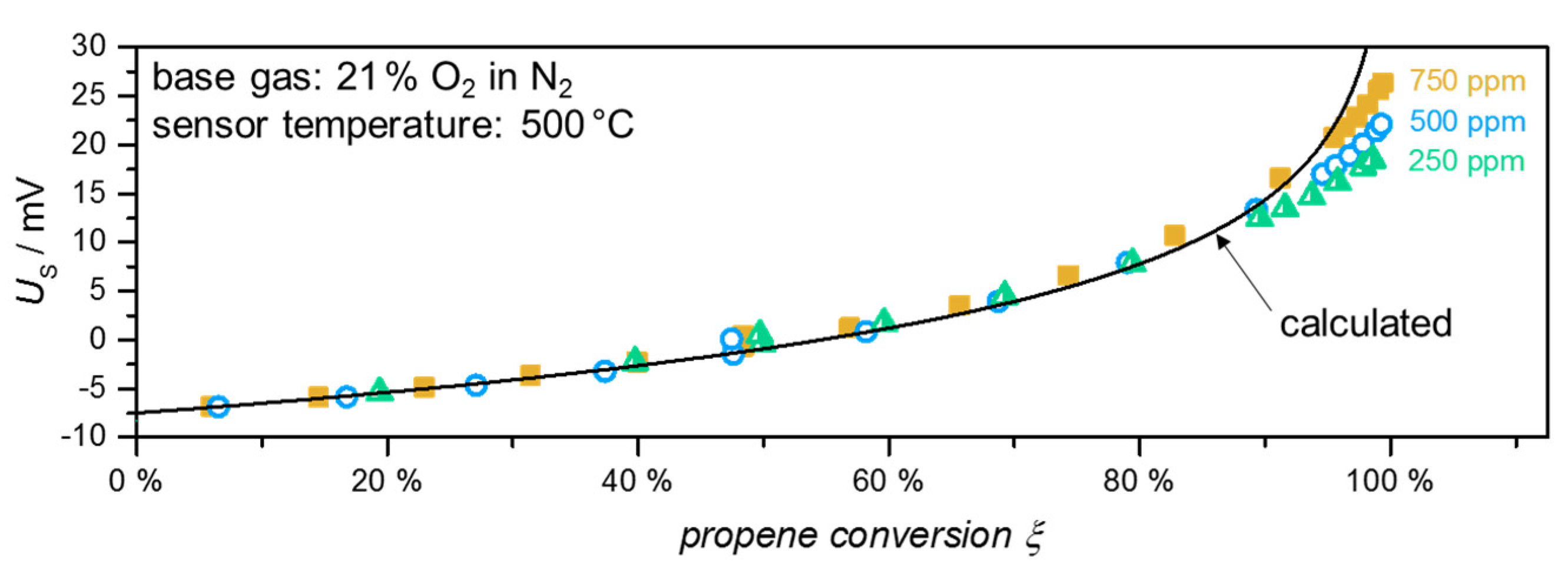
3. Experimental Results
Conflicts of Interest
References
- Van Nieuwstadt, M.; Upadhyay, D.; Yuan, F. Diagnostics for Diesel Oxidation Catalysts. SAE Tech. Paper 2005, 2005-01-3602. [Google Scholar] [CrossRef]
- Beulertz, G.; Votsmeier, M.; Moos, R. In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies. Appl. Sci. 2015, 5, 174–186. [Google Scholar] [CrossRef]
- Posada, F.; Bandivadekar, A. Global Overview of On-Board Diagnostics (OBD) Systems for Heavy-Duty Vehicles. The International Council on Clean Transportation (ICCT). Available online: http://theicct.org/sites/default/files/publications/ICCT_Overview_OBD-HDVs_20150209.pdf (accessed on 9 June 2017).
- Biskupski, D.; Geupel, A.; Wiesner, K.; Fleischer, M.; Moos, R. Platform for a hydrocarbon exhaust gas sensor utilizing a pumping cell and a conductometric sensor. Sensors 2009, 9, 7498–7508. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.; Dubbe, A.; Fischerauer, G.; Moos, R. Thick-film impedance based hydrocarbon detection based on chromium(III) oxide/zeolite interfaces. Sens. Actuators B 2006, 118, 73–77. [Google Scholar] [CrossRef]
- Hagen, G.; Leupold, N.; Wiegärtner, S.; Moos, R. Sensor Tool for Fast Catalyst Material Characterization. Top. Catal. 2017, 60, 312–317. [Google Scholar] [CrossRef]
- Park, N.-H.; Akamatsu, T.; Itoh, T.; Izu, N.; Shin, W. Calorimetric thermoelectric gas sensor for the detection of hydrogen, methane and mixed gases. Sensors 2014, 14, 8350–8362. [Google Scholar] [CrossRef] [PubMed]
- Sahner, K.; Fleischer, M.; Magori, E.; Meixner, H.; Deerberg, J.; Moos, R. HC-sensor for exhaust gases based on semiconducting doped SrTiO3 for On-Board Diagnosis. Sens. Actuators B 2006, 114, 861–868. [Google Scholar] [CrossRef]
- Schmidt-Zhang, P.; Guth, U. A planar thick film sensor for hydrocarbon monitoring in exhaust gases. Sens. Actuators B 2004, 99, 258–263. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Kysar, J.; Brosha, E.L.; Kreller, C.R. Development and testing of an electrochemical methane sensor. Sens. Actuators B 2016, 228, 162–167. [Google Scholar] [CrossRef]
- Wiegärtner, S.; Hagen, G.; Kita, J.; Reitmeier, W.; Hien, M.; Grass, P.; Moos, R. Thermoelectric hydrocarbon sensor in thick-film technology for on-board-diagnostics of a diesel oxidation catalyst. Sens. Actuators B 2015, 214, 234–240. [Google Scholar] [CrossRef]
- Wu, M.-C.; Micheli, A.L. Calorimetric hydrocarbon sensor for automotive exhaust applications. Sens. Actuators B 2004, 100, 291–297. [Google Scholar] [CrossRef]
- Zosel, J.; Westphal, D.; Jakobs, S.; Müller, R.; Guth, U. Au–oxide composites as HC-sensitive electrode material for mixed potential gas sensors. Solid State Ion. 2002, 152–153, 525–529. [Google Scholar] [CrossRef]
- Moos, R. A Brief Overview on Automotive Exhaust Gas Sensors Based on Electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2, 401–413. [Google Scholar] [CrossRef]
- Riegel, J. Exhaust gas sensors for automotive emission control. Solid State Ion. 2002, 152–153, 783–800. [Google Scholar] [CrossRef]
- Ritter, T.; Hagen, G.; Kita, J.; Wiegärtner, S.; Schubert, F.; Moos, R. Self-heated HTCC-based ceramic disc for mixed potential sensors and for direct conversion sensors for automotive catalysts. Sens. Actuators B 2017, 248, 793–802. [Google Scholar] [CrossRef]
- Guth, U.; Zosel, J. Electrochemical solid electrolyte gas sensors—Hydrocarbon and NOx analysis in exhaust gases. Ionics 2004, 10, 366–377. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Fergus, J.W. Sensing mechanism of non-equilibrium solid-electrolyte-based chemical sensors. J. Solid State Electrochem. 2011, 15, 971–984. [Google Scholar] [CrossRef]
- Hagen, G.; Burger, K.; Wiegärtner, S.; Schönauer-Kamin, D.; Moos, R. A mixed potential based sensor that measures directly catalyst conversion—A novel approach for catalyst on-board diagnostics. Sens. Actuators B 2015, 217, 158–164. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ritter, T.; Hagen, G.; Moos, R. Direct Catalyst Conversion Sensor in Form of a Single Self-Heated Mixed-Potential Device. Proceedings 2017, 1, 424. https://doi.org/10.3390/proceedings1040424
Ritter T, Hagen G, Moos R. Direct Catalyst Conversion Sensor in Form of a Single Self-Heated Mixed-Potential Device. Proceedings. 2017; 1(4):424. https://doi.org/10.3390/proceedings1040424
Chicago/Turabian StyleRitter, Thomas, Gunter Hagen, and Ralf Moos. 2017. "Direct Catalyst Conversion Sensor in Form of a Single Self-Heated Mixed-Potential Device" Proceedings 1, no. 4: 424. https://doi.org/10.3390/proceedings1040424
APA StyleRitter, T., Hagen, G., & Moos, R. (2017). Direct Catalyst Conversion Sensor in Form of a Single Self-Heated Mixed-Potential Device. Proceedings, 1(4), 424. https://doi.org/10.3390/proceedings1040424





