A Textile Based Polypyrrole Chloride Sensor for Agricultural Use †
Abstract
:1. Introduction
2. Sensor Fabrication and Testing
4. Results and Discussion
5. Conclusions and Future Plan
Acknowledgments
Conflicts of Interest
References
- Guth, U.; Vonau, W.; Zosel, J. Recent developments in electrochemical sensor application and technology—A review. Meas. Sci. Technol. 2009, 20, 1–14. [Google Scholar] [CrossRef]
- Klaus, J.; Smettem, K.; Pfister, L.; Harris, N. Solute transport in streams of varying morphology inferred from a high resolution network of potentiometric wireless chloride sensors. In Proceedings of the EGU 2017, Vienna, Austria, 23–28 April 2017. [Google Scholar]
- Harris, N.; Cranny, A. Rivers, M.; Smettem, K.; Barrett-Lennard, E.G. Application of distributed wireless chloride sensors to environmental monitoring: Initial results. IEEE Trans. Instrum. Meas. 2016, 65, 736–743. [Google Scholar] [CrossRef]
- Cranny, A.; Harris, N.; White, N. Screen-printable porous glass: A new material for electrochemical sensors J. Mater. Sci. Mater. Electron. 2015, 26, 4557–4564. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kumar, P.; Park, D.S.; Shim, Y.B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Romero, G.A.; Palomar-Pardavé, M.E.; Ramrez-Silva, M.T. Development of a novel nitrate-selective composite sensor based on doped polypyrrole. Anal. Bioanal. Chem. 2007, 387, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Chatterjee, A. Polyoyrrole based electro-conductive cotton yarn. J. Text. Sci. Eng. 2014, 4, 1–4. [Google Scholar]
- Najar, S.S.; Kaynak, V.; Foitzik, C.R. Conductive wool yarns by continuous vapour phase polymerization of pyrrole. Synth. Met. 2007, 157, 1–4. [Google Scholar] [CrossRef]
- Cranny, A.; Harris, N.R.; Nie, M.; Wharton, J.A.; Wood, R.J.; Stokes, K.R. Screen-printed potentiometric Ag/AgCl chloride sensors: Lifetime performance and their use in soil salt measurements. Sens. Actuators A Phys. 2011, 169, 288–294. [Google Scholar] [CrossRef]
- Glanc, M.; Sophocleous, M.; Atkinson, J.K.; Garcia-Breijo, E. The effect on performance of fabrication parameter variations of thick-film screen printed silver/silver chloride potentiometric reference electrodes. Sens. Actuators A Phys. 2013, 197, 1–8. [Google Scholar] [CrossRef]
- Glanc-Gostkiewicz, M. An investigation of thick-film electrodes for environmental monitoring. Ph.D. Thesis, University of Southampton, Southampton, UK, December 2016. [Google Scholar]
| Sensor | Potential (V) | Pyrrole (M) | KCl (M) | Volume (mL) | Duration (h) |
|---|---|---|---|---|---|
| 1 | 0.85 | 0.4 | 1.0 | 100 | 1 |
| 2 | 0.85 | 2 | |||
| 3 | 0.75 | 1 | |||
| 4 | 0.75 | 2 |
| Sensor | Electrode Sides Length (mm) | Total Area (mm2) | Barrier Area (mm2) | Sensing Area (mm2) | ||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | d | b × c | b × d | a × b | b × c | |
| 1 | 28 | 8 | 36 | 10 | 288 | 80 | 224 | - |
| 2 | 30 | 7 | 38 | 10 | 266 | 70 | 210 | - |
| 3 | 28 | 13 | 38 | 5 | 494 | 65 | 364 | - |
| 4 | - | 11 | 29 | - | 319 | - | - | 319 |
| PPy(Cl−) Sensor | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| Sensitivity Tests | Solution Conc. | Solution Conc. | Solution Conc. | ||||
| 0.1 M–0.01 M | 0.01 M–1 M | 1 M–0.1 M | 0.1 M–0.01 M | 1 M–0.1 M | 0.1 M–0.01 M | ||
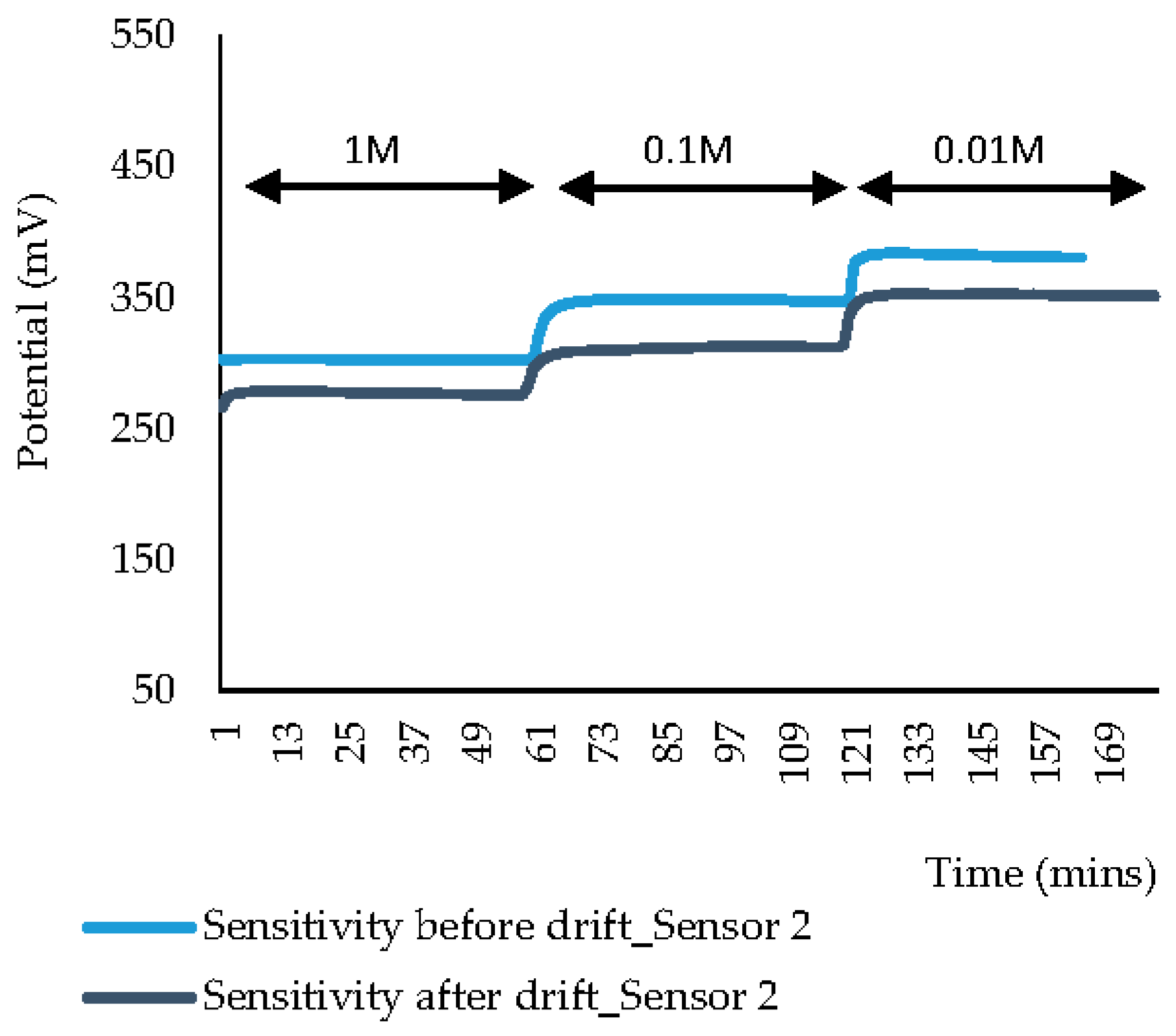
| Before drift (mv/decade) | Test 1 | 47.3 | 107.5 | 45.0 | 38.1 | 23.2 | 271.7 |
| Test 2 | 27.9 | 80.4 | 47.6 | 45.2 | not recorded | ||
| After drift (mv/decade) | Test 3 | 25.4 | 18.1 | 33.1 | 41.3 | 38.2 | 30.3 |
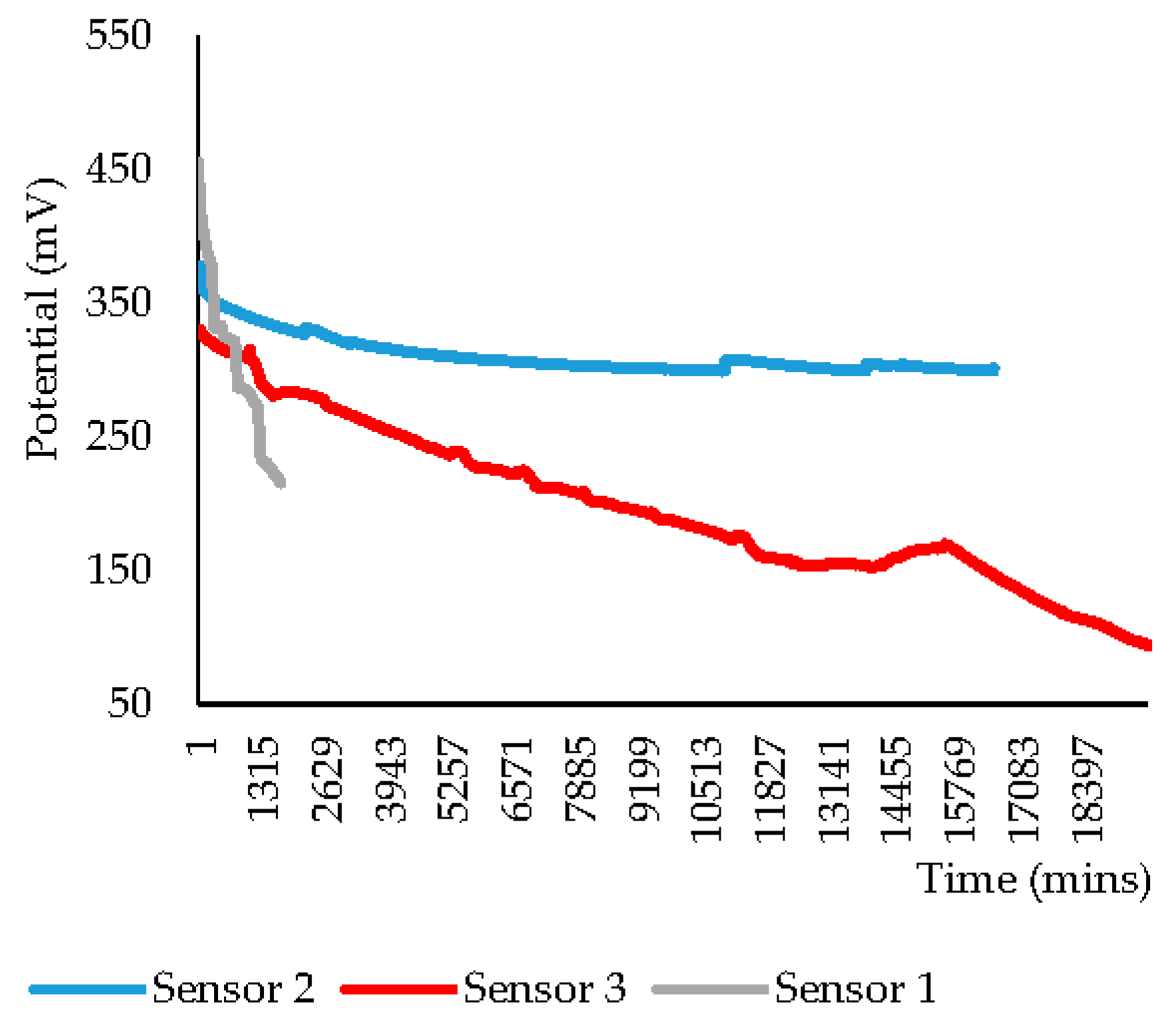
| PPy(Cl−) Sensor | Drift Duration | Barrier Layer | Initial Potential | Final Potential | Difference |
|---|---|---|---|---|---|
| 1 | 8 days | Yes | 455.7 | 147.5 | 308.2 |
| 2 | 13 days | 326.7 | 299.7 | 27.0 | |
| 3 | 15 days | 326.6 | 93.6 | 232.4 | |
| 4 | 25 min | No | 439.3 | 179.2 | 260.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glanc-Gostkiewicz, M.; Harris, N. A Textile Based Polypyrrole Chloride Sensor for Agricultural Use. Proceedings 2017, 1, 430. https://doi.org/10.3390/proceedings1040430
Glanc-Gostkiewicz M, Harris N. A Textile Based Polypyrrole Chloride Sensor for Agricultural Use. Proceedings. 2017; 1(4):430. https://doi.org/10.3390/proceedings1040430
Chicago/Turabian StyleGlanc-Gostkiewicz, Monika, and Nick Harris. 2017. "A Textile Based Polypyrrole Chloride Sensor for Agricultural Use" Proceedings 1, no. 4: 430. https://doi.org/10.3390/proceedings1040430
APA StyleGlanc-Gostkiewicz, M., & Harris, N. (2017). A Textile Based Polypyrrole Chloride Sensor for Agricultural Use. Proceedings, 1(4), 430. https://doi.org/10.3390/proceedings1040430




