Abstract
The aim of this work is to investigate the mechanisms of nitrate and nitrite ions electro-reduction in neutral solution at boron doped diamond (BDD) electrodes modified with metal catalyst nanoparticles. The electrode preparation consists in sputtering a thin metal layer onto polycrystalline BDD by a physical vapor deposition method, followed by a dewetting heat treatment at 700 °C under oxygen-free atmosphere. Such a process leads to a stable population of nanosized metal particles as characterized by scanning electron microscopy (SEM) and cyclic voltammetry. Electro-reduction of NO2− and NO3− was characterized both on a bare BDD electrode and on BDD electrodes decorated with platinum-gold, ruthenium-gold and ruthenium nanoparticles in the range 4 to 25 mM. The amperometric response was enhanced by the presence of the nanoparticles, the most sensitive electrode being Pt-Au/BDD and Ru-Au/BDD for nitrite and nitrate ions, respectively.
1. Introduction
Nitrate and nitrite ions are important in human health and environmental chemistry. Therefore, they need to be monitored carefully to ensure that they do not exceed the thresholds established by the World Health Organization [1]. Many methods have been developed for the determination of nitrate and nitrite concentrations. Those based on optical spectroscopy and ion chromatography are the most common ones [2]. Additionnally, electrochemical approaches have been investigated using various reactive electrodes, thus bringing several advantages including rapid responses, simplicity of use, reliability and high sensitivity. In this context, the use of Boron Doped Diamond (BDD) as working electrode material features major advantages over other materials: a wide potential window in aqueous media (> 3 V), low background current, and long-term stability. BDD electrodes have been reported for the detection nitrite/nitrate [3]. However, doped diamond exhibit relatively poor surface catalytic behavior, which may affect its reactivity toward these ions. This issue can be partially overcome by the immobilization of catalysts at the electrode surface such as specific enzymes [4]. Recently, it was also demonstrated that the deposition of transition metal nanoparticles over BDD electrode enhances their reactivity toward some analytes [5]. Beside, synergistic electrochemical effects in terms of catalytic activities can often be obtained when two or more metallic species are mixed together on the surface of the BDD electrodes [6]. Metal nanoparticles can be formed by various deposition methods including sputtering techniques [6], self-assembly (electrostatic interaction) [7] as well as electrochemical deposition procedures [8]. A promising, fast and convenient way to decorate conducting BDD surfaces with well-defined amounts of metal transition is using physical vapor deposition (PVD) techniques. Thus, in this work, metal alloy NPs were prepared by a PVD process on BDD electrodes and investigated for the enhanced electro-catalytic reduction of NO3− or NO2− [9]. Three electrode types were prepared and compared in terms of electrochemical performances for the detection of nitrite/nitrate: platinum-gold, ruthenium-gold and ruthenium nanoparticles (NPs) decorated BDD electrodes.
2. Experimental
2.1. Chemicals
Sodium Nitrate (NaNO3), Sodium Nitrite (NaNO2), lithium perchlorate (LiClO4) and sodium chloride (NaCl) were purchased from Sigma-Aldrich, France. The supporting electrolyte was a 1 M NaCl solution prepared in ultra-pure deionized water from a Millipore Direct Q3-UV with a resistivity of 18.2 MΩ·cm.
2.2. Preparation of Metal Nanoparticle Modified BDD Electrodes
The BDD electrodes were grown on a highly doped <100> silicon substrate by Plasma Enhanced Chemical Vapor deposition using a process described elsewhere [10]. Metal catalyst nanoparticles of either Ru, Ru-Au, or Pt-Au were deposited in a two-step process aiming at homogeneous size and density particles distribution of the nanoparticles as well as good adhesion over the BDD electrodes. The first step consisted in sputtering a thin layer (approx. 3 nm thick) of metal by PVD in argon atmosphere (6 × 10−3 mbar) at 40 W of RF power. Then the second step involved the formation of nuclei by annealing the samples at high temperature under hydrogen plasma in a home-made diamond growth reactor, at a pressure of 40 mbar and microwave power 900 W for 10 min. At the end of the procedure, small metal NPs are observed under SEM over the diamond electrode surface.
2.3. Electrochemical Measurements
Electrochemical measurements were performed using a potentiostat Autolab PGSTAT302N equipped with the software Nova. A three-electrode system was used using metal NPs/BDD as working electrode, a bare Pt mesh as counter electrode and a Pt wire as pseudo-reference electrode.
3. Results
3.1. Morphologic and Electrochemical Characterization of BDD Decorated with Transition Metals Nano-Catalysts
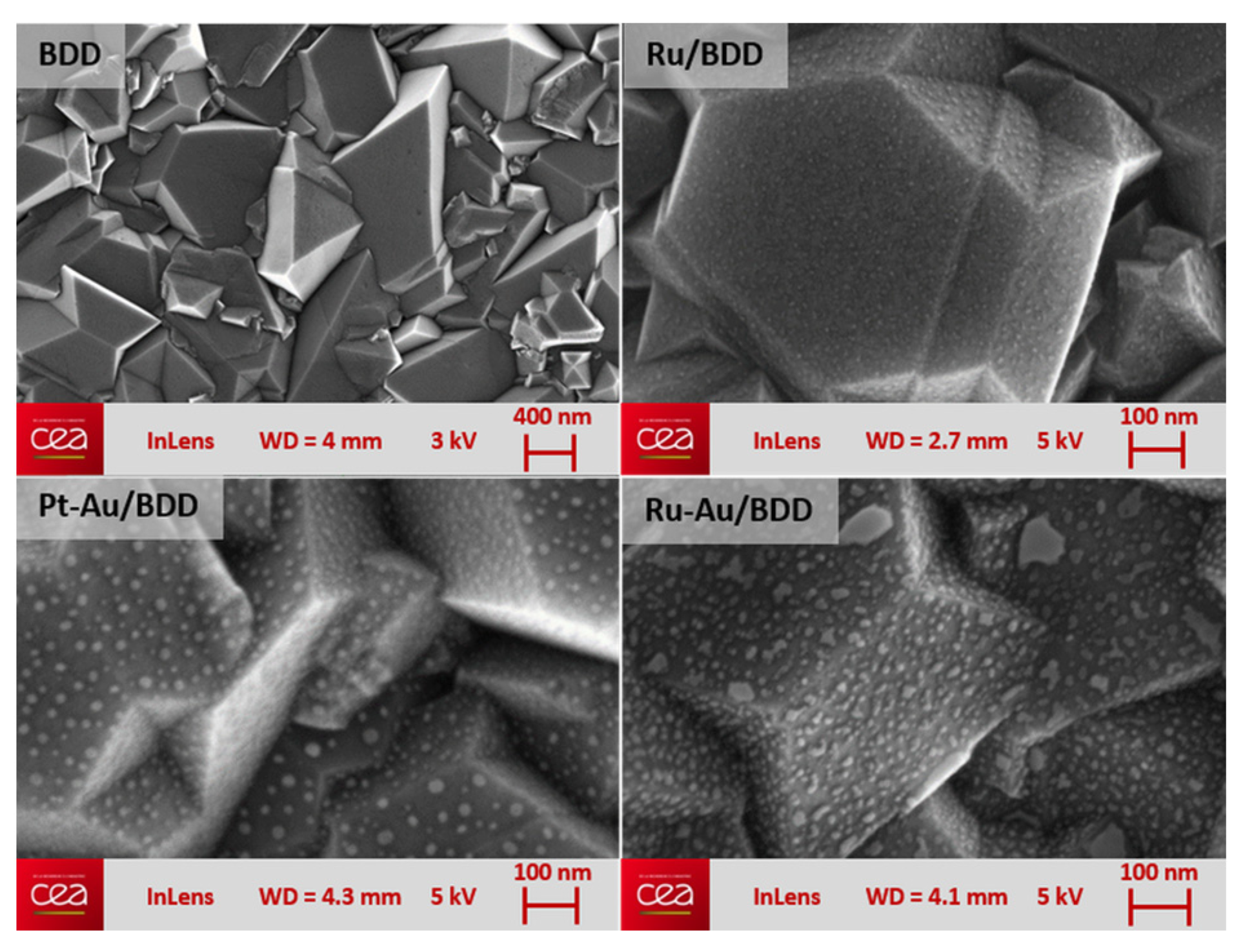
The PVD/dewetting process allowed obtaining metallic NPs onto BDD with a fairly uniform distribution across the entire BDD surface (Figure 1). It was found that the average size of the particles, which depends directly upon the amount of deposited metal, was between 2 and 30 nm. The particles were highly stable with good adhesion over the diamond surface: their stability was challenged through both the repetition of > 100 repeat amperometric electro-reduction measurements of nitrate/nitrite and the application of 100 current pulses of current density ±5 mA·cm−2, with no observable degradation of the electrodes. The resulting electrodes were characterized by cyclic voltammetry recorded in 0.1 M lithium perchlorate aqueous solution free of any electro-active redox species thus underlining the capacitive behavior of these electrodes along with their potential window in such electrolyte.
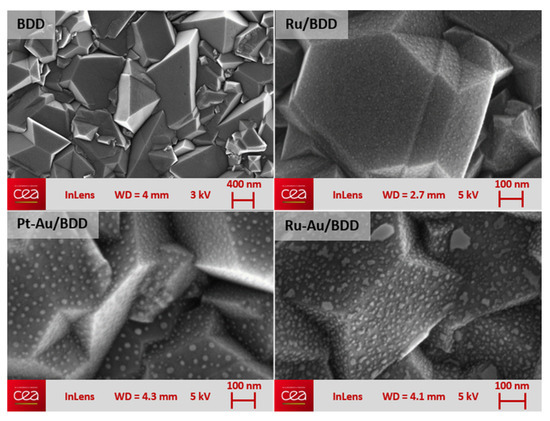
Figure 1.
SEM pictures of polycrystalline BDD electrode surface before and after metal NPs deposition.
The results, summarized in Table 1, revealed that the BDD electrodes coated with metal transition NPs exhibit low capacitive currents at 0 V where no faradic activity was observed. The current values are only slightly higher than those of the bare BDD electrode, and much lower than the values expected for bulk metal electrodes. The potential window of the modified electrodes is reduced when compared to the bare BDD electrode, but it remains wide, above 3 V for three of the four modified electrodes. Overall these properties of the modified electrodes are found to be lying between those of bare diamond and those of bare metal electrodes, respectively. Hence the new electrodes offer a good compromise between bare diamond are bare metal electrodes, featuring advantages from both electrodes: stability, wide potential window, and low background current on one side, and catalytic properties on the other side, respectively.

Table 1.
Potential window recorded within the range of current threshold ± 100 µA and capacitive current recorded at 0 V vs. Pt for the bare diamond electrode and the BDD electrodes modified with metal nanoparticles.
3.2. Nitrate and Nitrite Detection
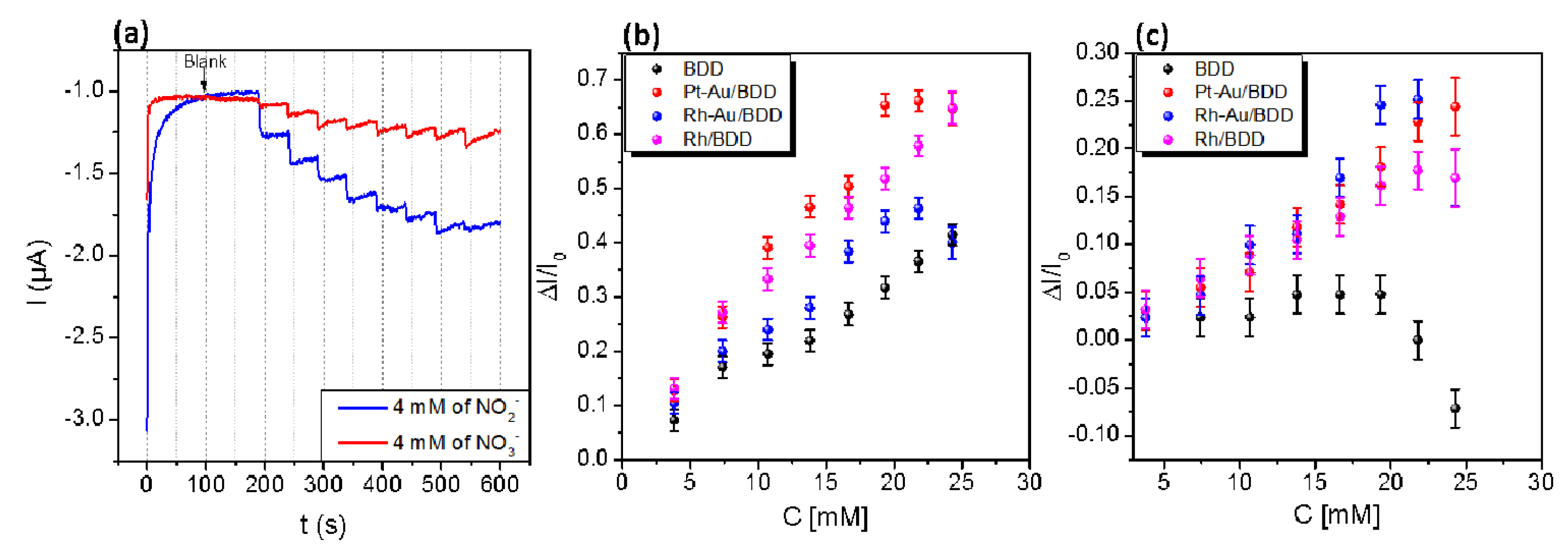
The amperometric detection of both nitrate and nitrite was assessed by chronoamperometry. Figure 2a shows the typical chronoamperometric response of a Pt-Au/BDD electrode to successive additions of 1 mL solutions of 4 mM nitrate (red curve) and 4 mM nitrite (blue curve), into continuously stirred 1 M NaCl aqueous electrolyte solution, respectively. For both ions a significant cathodic current response is observed. As expected, the sensitivity toward nitrite appears to be higher than toward nitrate since it is well accepted that the latter is difficult to reduce in neutral media.
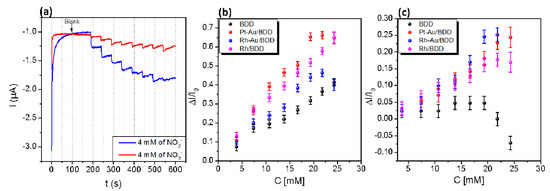
Figure 2.
(a) Chronoamperometric response of Pt-Au/BDD electrodes for successive addition of 1 mL of 0.1 mM nitrite or nitrate solutions into continuously stirred aqueous medium (300 rpm) of 1 M of NaCl under a potential of—1V vs. (Pt.) with a volume of 25 mL; Calibration curve of nitrite (b) and nitrate (c) detection.
The response of all electrodes to nitrite is fairly linear in the range 0–25 mM, with the sensitivity increasing in the order: BDD < Ru-Au/BDD < Ru/BDD < Pt-Au/BDD. The response to nitrate is linear in the range 0–20 mM for all electrodes but the BDD electrode. The sensitivity to nitrate increases in the following order: BDD < Ru/BDD < Pt-Au/BDD < Ru-Au/BDD. The BDD electrode exhibits the lowest sensitivity. Indeed, since the BDD surface is not favorable for adsorption, electrode processes that involve adsorbed intermediates are hindered on diamond, and many outer-sphere redox reactions require large overpotential above the equilibrium potential to proceed efficiently [11]. Here nitrate and nitrite reduction processes involving adsorbates such as NO or N2O, as observed on bulk transition metal electrodes [12], are expected to be favored due to the presence of the nanoparticles over the modified electrodes, which enhances their sensitivity as part of catalytic processes involving metal centers.
4. Conclusions
A range of transition metal catalysts were deposited successfully over BDD electrode surfaces using a physical deposition process, achieving good size distribution and high stability and adhesion of the particles. The electrochemical characteristics of the modified electrodes in terms of electrochemical capacitance and potential window were found to be a good compromise between bare BDD electrodes and bare metal electrodes, thus enabling to benefit from the catalytic properties of transition metals while keeping to some extents the features of BDD electrodes in terms of stability and electrochemical assets. The metal nanoparticles were found to enhance the electrocatalytic response of the modified BDD electrodes toward nitrite and nitrate reduction. The best electrode for nitrite was found to be Pt-Au/BDD and for nitrate Ru-Au/BDD, respectively. It is now envisaged to use electrode arrays of such electrodes and appropriate multivariate analysis to improve selectivity between both ions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; American Public Health Association, American Water Works Association: Washington, DC, USA, 2012. [Google Scholar]
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and Determination of Nitrate and Nitrite: A Review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Bouamrane, F.; Tadjeddine, A.; Butler, J.E.; Tenne, R. Electrochemical Study of Diamond Thin Films in Neutral and Basic Solutions of Nitrate. J. Electroanal. Chem. 1996, 405, 95–99. [Google Scholar] [CrossRef]
- Minami, T.; Sasaki, Y.; Minamiki, T.; Wakida, S.; Kurita, R. Selective Nitrate Detection by an Enzymatic Sensor Based on an Extended-Gate Type Organic Fi Eld-Effect Transistor. Biosens. Bioelectron. 2016, 81, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Belghiti, D.K.; Scorsone, E.; Bergonzo, P. Boron Doped Diamond/Metal Nanoparticle Catalysts Hybrid Electrode Array for the Detection of Pesticides in Tap Water. Procedia Eng. 2016, 168, 428–431. [Google Scholar] [CrossRef]
- Roustom, B.E.; Foti, G.; Comninellis, C. Preparation of Gold Nanoparticles by Heat Treatment of Sputter Deposited Gold on Boron-Doped Diamond Film Electrode. Electrochem. Commun. 2005, 7, 398–405. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, S.; Lee, J.; Kim, J.; Lim, D. Electrochemical Sensor Based on Au Nanoparticles Decorated Boron-Doped Diamond Electrode Using Ferrocene-Tagged Aptamer for Proton Detection. J. Electroanal. Chem. 2012, 680, 139–144. [Google Scholar] [CrossRef]
- Brylev, O.; Sarrazin, M.; Bélanger, D.; Roué, L. Rhodium Deposits on Pyrolytic Graphite Substrate: Physicochemical Properties and Electrocatalytic Activity towards Nitrate Reduction in Neutral Medium. Appl. Catal. B Environ. 2006, 64, 243–253. [Google Scholar] [CrossRef]
- Vanhove, E.; de Sanoit, J.; Mailley, P.; Pinault, M.-A.; Jomard, F.; Bergonzo, P. High Reactivity and Stability of Diamond Electrodes: The Influence of the B-doping Concentration. Phys. Status Solidi A 2009, 206, 2063–2069. [Google Scholar] [CrossRef]
- Holt, K.B.; Ziegler, C.; Caruana, D.J.; Zang, J.; Millan-Barrios, E.J.; Hu, J.; Foord, J.S. Redox Properties of Undoped 5 Nm Diamond Nanoparticles. Phys. Chem. Chem. Phys. 2008, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Duca, M.; Kavvadia, V.; Rodriguez, P.; Lai, S.C.S.; Hoogenboom, T.; Koper, M.T.M. New Insights into the Mechanism of Nitrite Reduction on a Platinum Electrode. J. Electroanal. Chem. 2010, 649, 59–68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).