1. Introduction
Water vapor is the most important factor to influence on gas sensing properties. We can obtain high gas sensor response in dry atmosphere, but the gas response is decreased gradually by introducing water vapor. Such phenomena are strongly related with oxygen adsorption species on SnO2 particles. Previously we reported that oxygen adsorption species is O2− and O− in dry and wet atmosphere, respectively [1]. However, the effect of treatment before measurement doesn’t have discussed. In this study, we investigated pretreatment of neat SnO2 and Sb-doped SnO2 for behavior of oxygen adsorption.
2. Experiments
In this study, we used the following equation for analysis of oxygen adsorption species.
R is electric resistance (Ω) in measurement condition, R0 electric resistance (Ω) at flat band, a crystalline size (nm), ND donor density (nm−3), PO2 oxygen partial pressure (atm), K1 and K2 adsorption equilibrium constants for O− (nm2/atm) and O2− (nm8/atm), respectively, and c constant.
We used SnO2 and Sb(0.1 mol.%)-SnO2 particles calcined at 600–700 °C in various atmosphere. After the powders were deposited on alumina substrates with Au electrodes by screen-printing, the elements were calcined at 580 °C in oxygen atmosphere for 3 h. After that, the resulting sensor elements were pretreated at 580 °C for 3 h in N2, 0.3%O2, 1%O2 or 100%O2. The sensor element was cooled to 350 °C with keeping the atmosphere of the pretreatment, and the oxygen partial pressure dependence of the electrical resistance was measured in dry and wet atmosphere (PH2O = 0.03 atm).
3. Results and Discussion
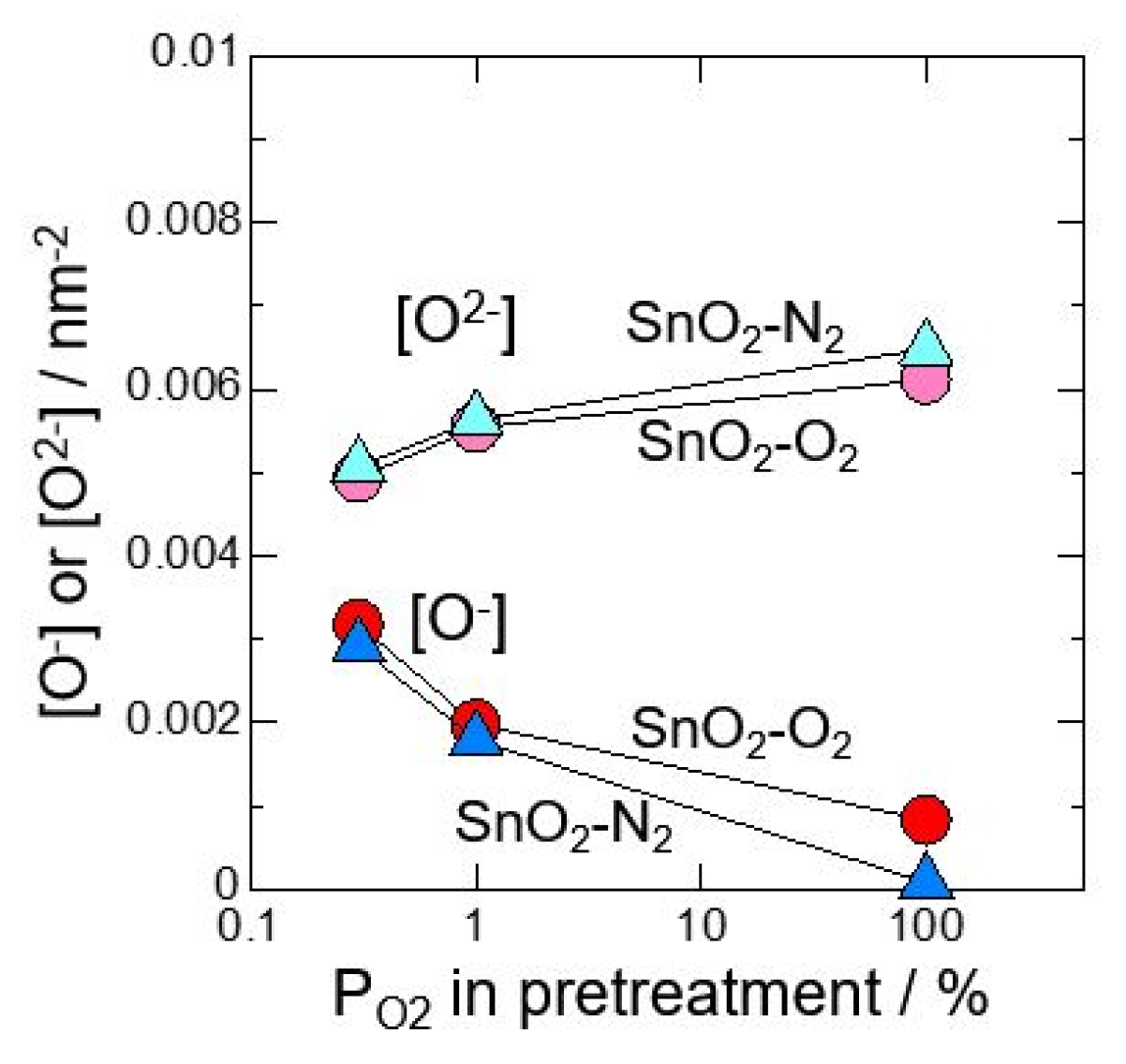
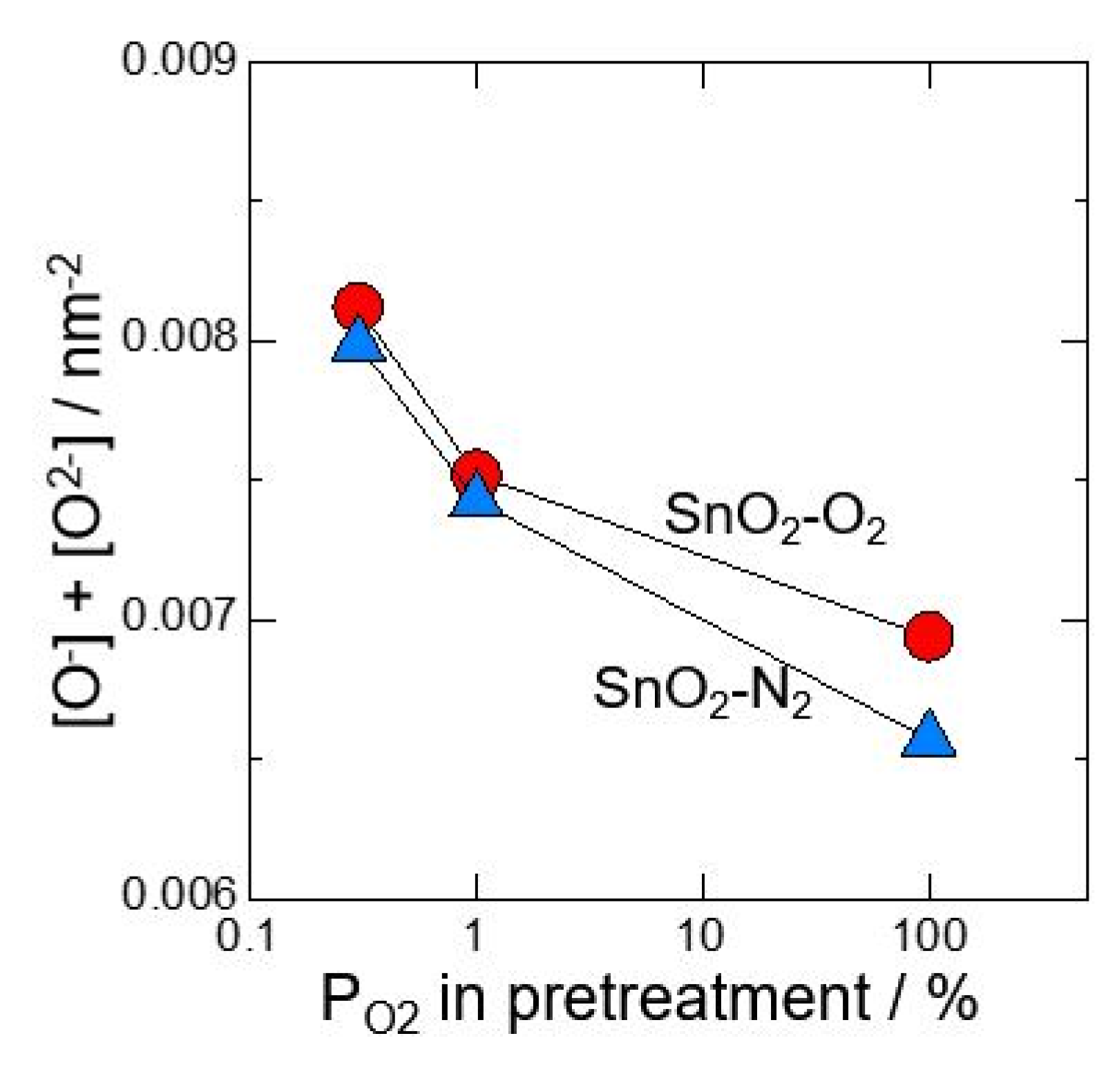
At first, neat SnO2 calcined at 700 °C in O2 or N2 atmosphere was investigated after pretreating at 580 °C for 3 h in 0.3%O2, 1%O2 or 100%O2. Figure 1 shows relationship between amount of oxygen adsorption species and oxygen partial pressure in pretreatment. It was revealed that O2− adsorbs with precedence than O−. In addition, by reducing oxygen partial pressure at pretreatment, the amount of O2− adsorption decreased and the amount of O- adsorption increased. Figure 2 shows total amounts of [O2−] and [O−] against oxygen partial pressure in pretreatment. It is found that the total amount of oxygen adsorption increases by reducing oxygen partial pressure at pretreatment. Furthermore, it is clear that the control of atmosphere at pretreatment gives a big influence on amounts of oxygen adsorption than the control of atmosphere at the powder calcination.
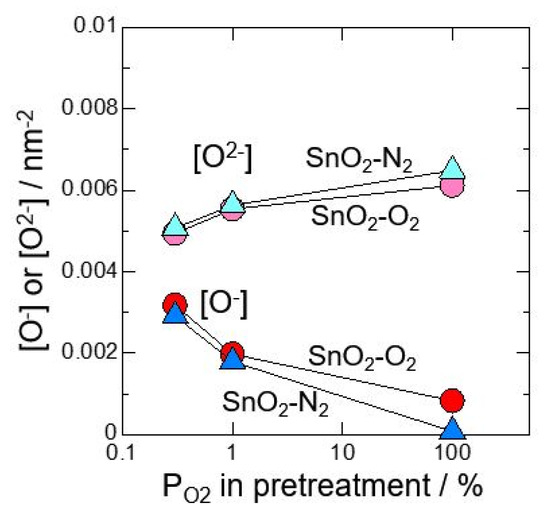
Figure 1.
Relationship between amount of oxygen adsorption species and oxygen partial pressure in pretreatment (measured in dry atmosphere).
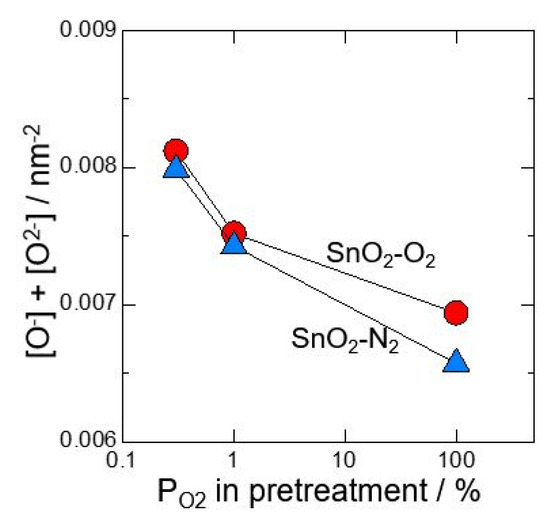
Figure 2.
Total amounts of [O2−] and [O−] against oxygen partial pressure in pretreatment (measured in dry atmosphere).
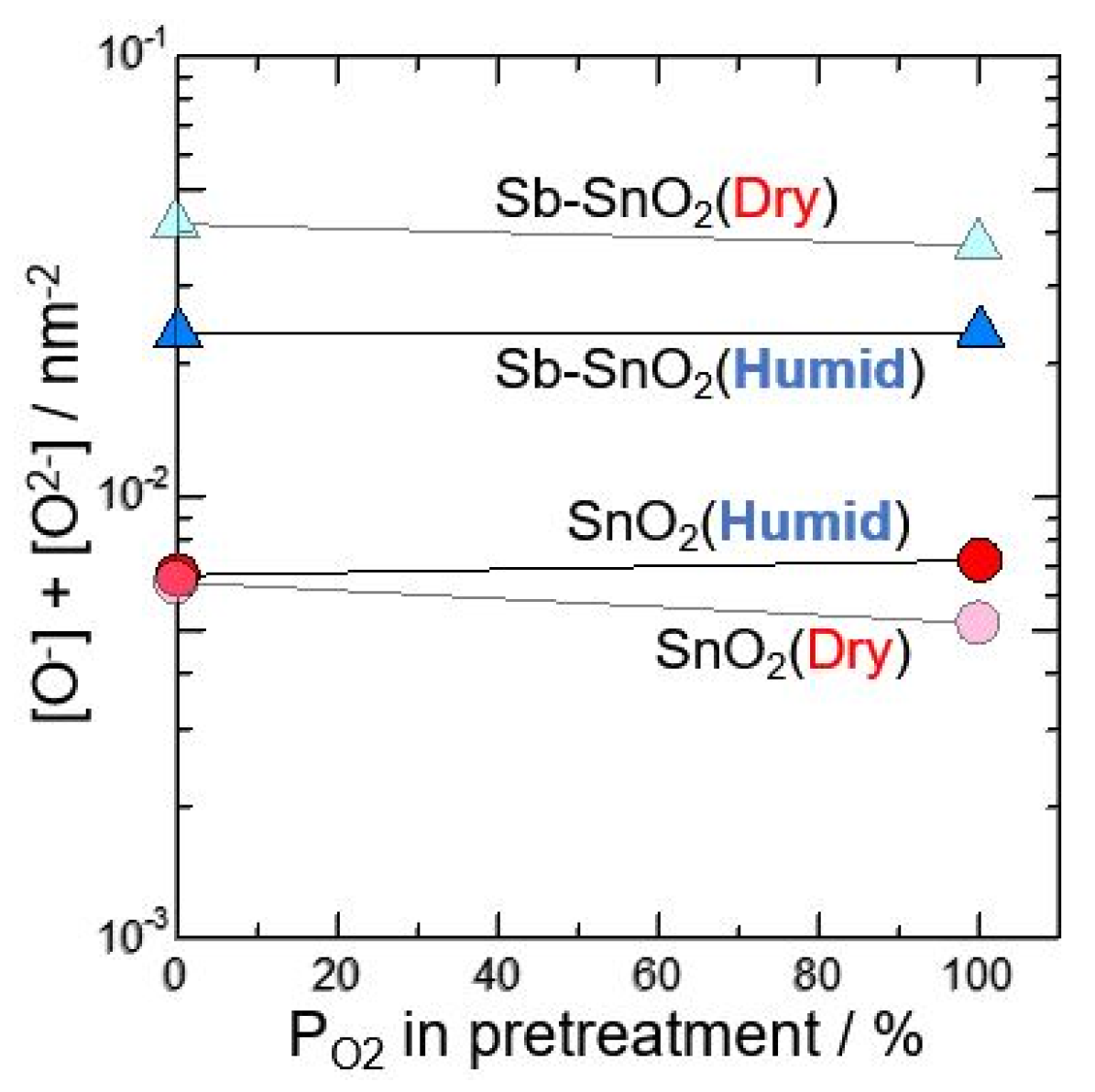
Second, neat SnO2 and Sb-doped SnO2 calcined at 600 °C in O2 atmosphere was investigated was investigated in wet atmosphere after pretreating at 580 °C for 3 h in N2 or 100%O2. Figure 3 shows total amounts of [O2−] and [O−] against oxygen partial pressure in pretreatment (measured in dry and wet atmosphere). The difference in the total amounts of oxygen adsorption species for dry and wet atmosphere was not observed, although Sb-SnO2 are more on the total amounts of oxygen adsorption than neat SnO2. From these results, it is considered that, at the first stage by changing dry to wet atmosphere, the hydroxyl group formation from water vapor may be related with the reduce in electric resistance. However, when the elements are kept for long time in wet atmosphere, the oxygen adsorption species changes O2− to O−, and the resulting also gives the reduce in electric resistance, as reported previously [1].
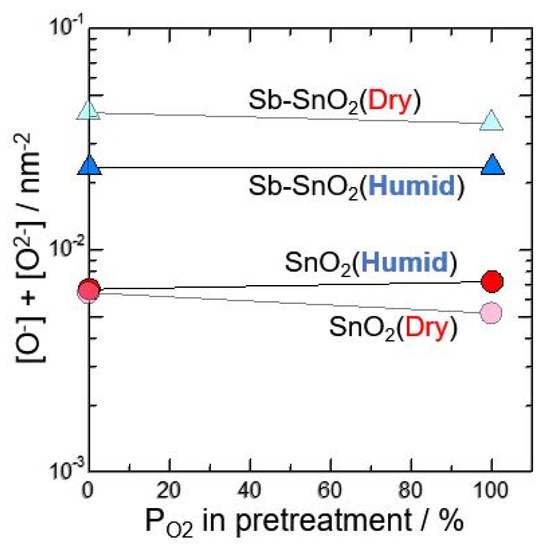
Figure 3.
Total amounts of [O2−] and [O−] against oxygen partial pressure in pretreatment (measured in dry and wet atmosphere).
Reference
- Yamazoe, N.; Suematsu, K.; Shimanoe, K. Extension of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens. Actuators B Chem. 2012, 163, 128–135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).