Surface Properties of SnO2 Nanolayers Deposited by Rheotaxial Growth and Vacuum Oxidation for Potential Gas Sensor Applications †
Aim
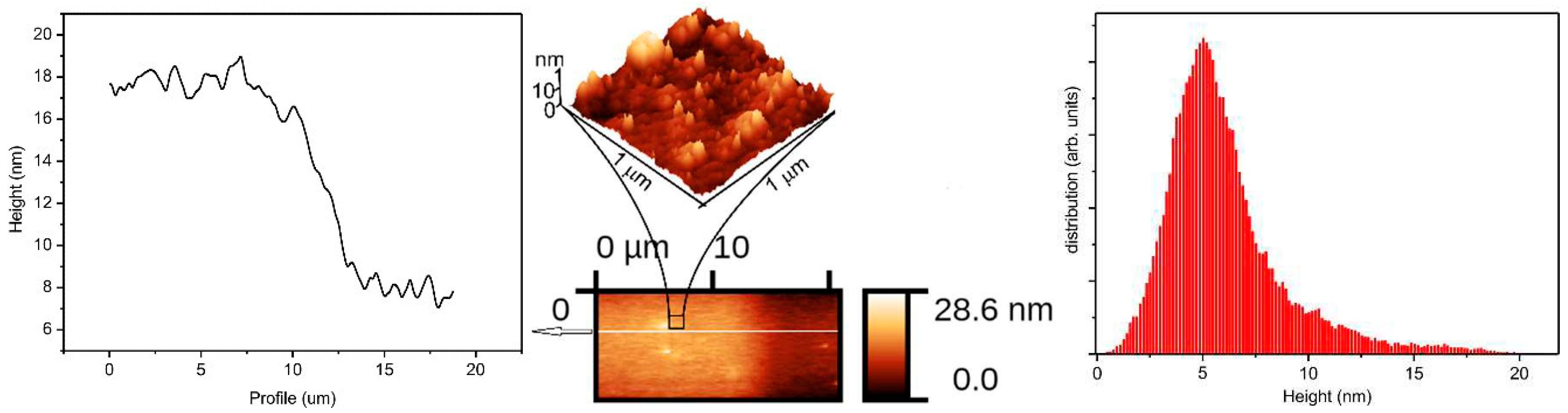
Results
Funding
Conflicts of Interest

| Samples | XPS lines RI | ARC | ||
|---|---|---|---|---|
| Substrates | RGVO SnO2 | O1s (a.u.) | Sn3d5/2 (a.u.) | [O]/[Sn] |
| Pure SiO2 | As-deposited | 47 | 340 | 0.90 |
| After oxidation | 48 | 336 | 0.93 | |
| SiO2 covered with Cr and Al | As-deposited | 46 | 328 | 0.95 |
| After oxidation | 50 | 320 | 1.02 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyson-Sypien, B.; Kwoka, M.; Krzywiecki, M. Surface Properties of SnO2 Nanolayers Deposited by Rheotaxial Growth and Vacuum Oxidation for Potential Gas Sensor Applications. Proceedings 2019, 14, 25. https://doi.org/10.3390/proceedings2019014025
Lyson-Sypien B, Kwoka M, Krzywiecki M. Surface Properties of SnO2 Nanolayers Deposited by Rheotaxial Growth and Vacuum Oxidation for Potential Gas Sensor Applications. Proceedings. 2019; 14(1):25. https://doi.org/10.3390/proceedings2019014025
Chicago/Turabian StyleLyson-Sypien, Barbara, Monika Kwoka, and Maciej Krzywiecki. 2019. "Surface Properties of SnO2 Nanolayers Deposited by Rheotaxial Growth and Vacuum Oxidation for Potential Gas Sensor Applications" Proceedings 14, no. 1: 25. https://doi.org/10.3390/proceedings2019014025
APA StyleLyson-Sypien, B., Kwoka, M., & Krzywiecki, M. (2019). Surface Properties of SnO2 Nanolayers Deposited by Rheotaxial Growth and Vacuum Oxidation for Potential Gas Sensor Applications. Proceedings, 14(1), 25. https://doi.org/10.3390/proceedings2019014025





