Evaluating the Performance of Different Commercial and Pre-Commercial Maize Varieties under Low Nitrogen Conditions Using Affordable Phenotyping Tools †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Remote Sensing and Proximal (Ground) Data Colleciton
2.3. Image Processing and Statistical Analyses
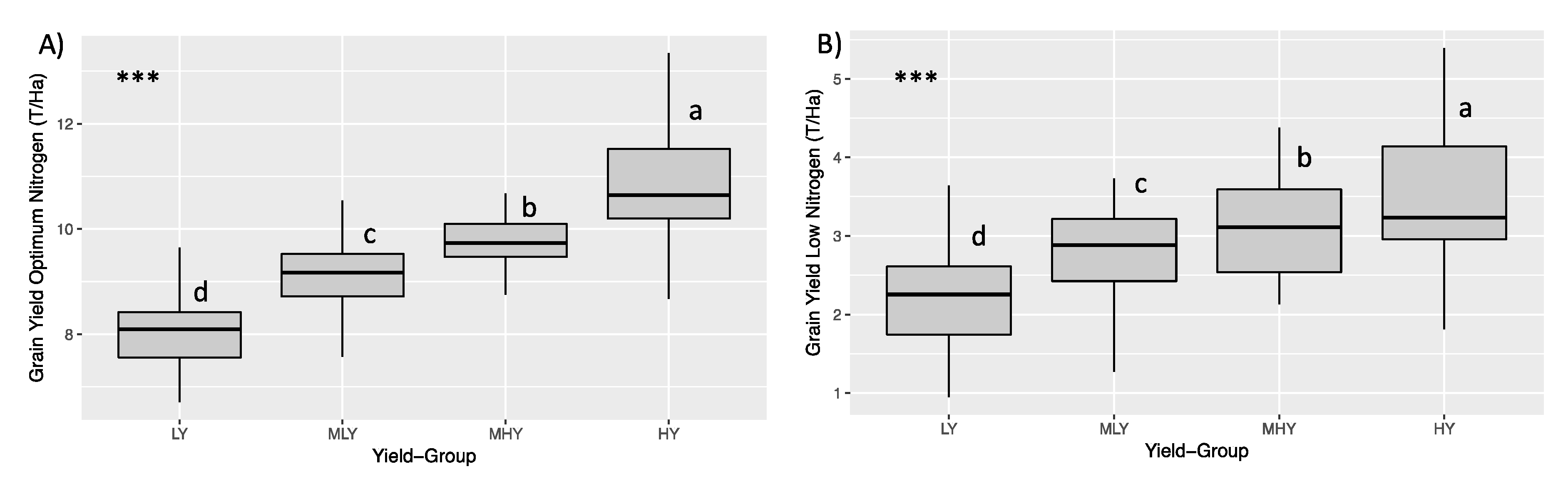
3. Results and Discussion
3.1. The Effect of Optimal Condition and Low Managed Nitrogen on Grain Yield
3.2. Performance of Remote Sensing Indices and Field Sensors in Estimating Grain Yield
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| NDVI | Normalized Difference Vegetation Index |
| HTTP | High Throughput Plant Phenotyping |
| RGB | Red-Green-Blue |
| GA | Green Area |
| GGA | Green Greener Area |
| CIMMYT | International Maize and Wheat Improvement Center |
| OP | Optimum Nitrogen |
| LOW | Low Managed Nitrogen |
| UAV | Unmanned Aerial Vehicle |
| ASI | Anthesis Silking Data |
| AD | Anthesis Data |
| PH | Plant Heigh |
| CSI | Crop Senescence Index |
| TGI | Triangular Greenness Index |
| NGRDI | Normalized Green Red Difference Index |
| HIS | Hue-Intensity-Saturation |
| LY | Low Yield |
| MLY | Medium Low Yield |
| MHY | Medium High Yield |
| HY | High Yield |
References
- FAO. Food and Agriculture Organization of the United Nations; Statistic Division. 2017. Available online: http://faostat.fao.org/ (accessed on 7 March 2018).
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Buerkert, A.; Bationo, A.; Piepho, H.P. Efficient phosphorus application strategies for increased crop production in sub-Saharan West Africa. Field Crops Res. 2001, 72, 1–15. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting Maize Productivity: Ideotypes Associated with Grain Yield under Drought Stress and Well-Watered Conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Reynolds, M.; Foulkes, J.; Furbank, R.; Griffiths, S.; King, J.; Murchie, E.; Parry, M.; Slafer, G. Achieving yield gains in wheat. Plant Cell Environ. 2012, 35, 1799–1823. [Google Scholar] [CrossRef]
- Debaeke, P.; Route, P.; Justes, E. Relationship between the normalize SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Rorie, R.L.; Purcell, L.C.; Morteza, M.; Karcher, D.E.; King, C.A.; Marsh, M.C.; Longer, D.E. Association of “greenness” in corn with yield and leaf nitrogen concentration. Agron. J. 2011, 103, 529–535. [Google Scholar] [CrossRef]
- Baenziger, M. Breeding for Drought and Nitrogen Stress Tolerance in Maize: From Theory to Practice; CIMMYT: Edo Mex, Mexico, 2000. [Google Scholar]
- Masuka, B.; Araus, J.L.; Das, B.; Sonder, K.; Cairns, J.E. Phenotyping for Abiotic Stress Tolerance in Maize. J. Integr. Plant Biol. 2012, 54, 238–249. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Ronald, B.; De Pauw, E. Evaluation of narrowband and broadband vegetation indices for determining optimal hyperspectral wavebands for agricultural crop characterization. Photogramm. Eng. Remote Sens. 2002, 68, 607–622. [Google Scholar]
- Vergara-Diaz, O.; Kefauver, S.C.; Elazab, A.; Nieto-Taladriz, M.T.; Araus, J.L. Grain yield losses in yellow-rusted durum wheat estimated using digital and conventional parameters under field conditions. Crop J. 2015, 3, 200–210. [Google Scholar] [CrossRef]
- Kefauver, S.C.; El-Haddad, G.; Vergara-Diaz, O.; Araus, J.L. RGB picture vegetation indexes for High-Throughput Phenotyping Platforms (HTPPs). In Proceedings of the SPIE Remote Sensing Conference, Toulouse, France, 21–24 September 2015. [Google Scholar]
- Casadesús, J.; Kaya, Y.; Bort, J.; Nachit, M.M.; Araus, J.L.; Amor, S.; Ferrazzano, G.; Maalouf, F.; Maccaferri, M.; Martos, V.; et al. Using vegetation indices derived from conventional digital cameras as selection criteria for wheat breeding in water-limited environments. Ann. Appl. Biol. 2007, 150, 227–236. [Google Scholar] [CrossRef]
- Zaman-Allah, M.; Vergara, O.; Araus, J.L.; Tarekegne, A.; Magorokosho, C.; Zarco-Tejada, P.J.; Cairns, J. Unmanned aerial platform-based multi-spectral imaging for field phenotyping of maize. Plant Methods 2015, 11, 35. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Hunt, E.R.; Cavigelli, M.; Daughtry, C.S.; Mcmurtrey, J.E.; Walthall, C.L. Evaluation of digital photography from model aircraft from remote sensing crop biomass and nitrogen status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Muruli, B.I.; Paulsen, G.M. Improvement of nitrogen use efficiency and its relationship to other traits in maize. Maydica 1981, 26, 63–73. [Google Scholar]
- Gallais, A.; Coque, M. Genetic variation and selection for nitrogen use efficiency in maize: A synthesis. Maydica 2005, 50, 531–537. [Google Scholar]
- Lukina, E.V.; Stone, M.L.; Raun, W.R. Estimating vegetation coverage in wheat using digital images. J. Plant Nutr. 1999, 22, 341–350. [Google Scholar] [CrossRef]
- Ding, L.; Wang, K.J.; Jiang, G.M.; Biswas, D.K.; Xu, H.; Li, L.F.; Li, Y.H. Effects of nitrogen deficiency on photosynthetic traits of maize hybrids released in different years. Ann. Bot. 2005, 96, 925–930. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral vegetation indices as nondestructive tools for determining durum wheat yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Hobbs, T.J. The use of NOAA-AVHRR NDVI data to assess herbage production in the arid rangelands of Central Australia. Int. J. Remote Sens. 1995, 16, 1289–1302. [Google Scholar] [CrossRef]
- Vetsch, J.A.; Randall, G.W. Corn production as affected by nitrogen application timing and tillage. Agron. J. 2004, 96, 502–509. [Google Scholar] [CrossRef]
- Chapman, S.; Baretto, H. Using a chlorophyll meter to estimate specific leaf nitrogen of tropical maize during vegetative growth. Agron. J. 1997, 89, 557–562. [Google Scholar] [CrossRef]
- Dwyer, L.M.; Anderson, A.M.; Ma, B.L. Stewart, D.W.; Tollenaar, M.; Gregorich, E. Quantifying the nonlinearity in chlorophyll meter response to corn leaf nitrogen concentration. Can. J. Plant Sci. 1995, 75, 179–182. [Google Scholar] [CrossRef]
- Masclaux, C.; Quilleré, I.; Gallais, A.; Hirel, B. The challenge of remobilization in plant nitrogen economy: A survey of physio-agronomic and molecular approaches. Ann. Appl. Biol. 2001, 138, 69–81. [Google Scholar] [CrossRef]
| RGB Indices/Aerial | R | p | RGB Indices/Ground | R | p | Additional Field Sensors | R | P |
|---|---|---|---|---|---|---|---|---|
| GGA | 0.1978 | *** | GGA | 0.2339 | *** | SPAD1 (18/02/16) | 0.2936 | *** |
| GA | 0.1659 | *** | GA | 0.2175 | *** | SPAD2 (01/03/16) | 0.2564 | *** |
| Hue | 0.1449 | *** | Hue | 0.2351 | *** | NDVI | 0.1404 | *** |
| Intensity | 0.0932 | *** | Intensity | 0.0090 | ||||
| Saturation | 0.1819 | *** | Saturation | 0.0515 | * | |||
| Lightness | 0.0848 | *** | Lightness | 0.0208 | * | |||
| a * | 0.1275 | *** | a * | 0.1467 | *** | |||
| b * | 0.1573 | *** | b * | 0.0080 | ||||
| u * | 0.1470 | *** | u * | 0.2021 | *** | |||
| v * | 0.0884 | *** | v * | 0.0002 | ||||
| CSI | 0.1830 | *** | CSI | 0.1031 | *** | |||
| TGI | 0.0527 | * | TGI | 0.0019 | ||||
| NGRDI | 0.1645 | *** | NGRDI | 0.0007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchaillot, M.L.; Gracia-Romero, A.; Zaman-Allah, M.A.; Tarekegne, A.; Prasanna, B.M.; Cairns, J.E.; Araus, J.L.; Kefauver, S.C. Evaluating the Performance of Different Commercial and Pre-Commercial Maize Varieties under Low Nitrogen Conditions Using Affordable Phenotyping Tools. Proceedings 2018, 2, 366. https://doi.org/10.3390/ecrs-2-05180
Buchaillot ML, Gracia-Romero A, Zaman-Allah MA, Tarekegne A, Prasanna BM, Cairns JE, Araus JL, Kefauver SC. Evaluating the Performance of Different Commercial and Pre-Commercial Maize Varieties under Low Nitrogen Conditions Using Affordable Phenotyping Tools. Proceedings. 2018; 2(7):366. https://doi.org/10.3390/ecrs-2-05180
Chicago/Turabian StyleBuchaillot, Ma. Luisa, Adrian Gracia-Romero, Mainassara A. Zaman-Allah, Amsal Tarekegne, Boddupalli M. Prasanna, Jill E. Cairns, Jose Luis Araus, and Shawn C. Kefauver. 2018. "Evaluating the Performance of Different Commercial and Pre-Commercial Maize Varieties under Low Nitrogen Conditions Using Affordable Phenotyping Tools" Proceedings 2, no. 7: 366. https://doi.org/10.3390/ecrs-2-05180
APA StyleBuchaillot, M. L., Gracia-Romero, A., Zaman-Allah, M. A., Tarekegne, A., Prasanna, B. M., Cairns, J. E., Araus, J. L., & Kefauver, S. C. (2018). Evaluating the Performance of Different Commercial and Pre-Commercial Maize Varieties under Low Nitrogen Conditions Using Affordable Phenotyping Tools. Proceedings, 2(7), 366. https://doi.org/10.3390/ecrs-2-05180





