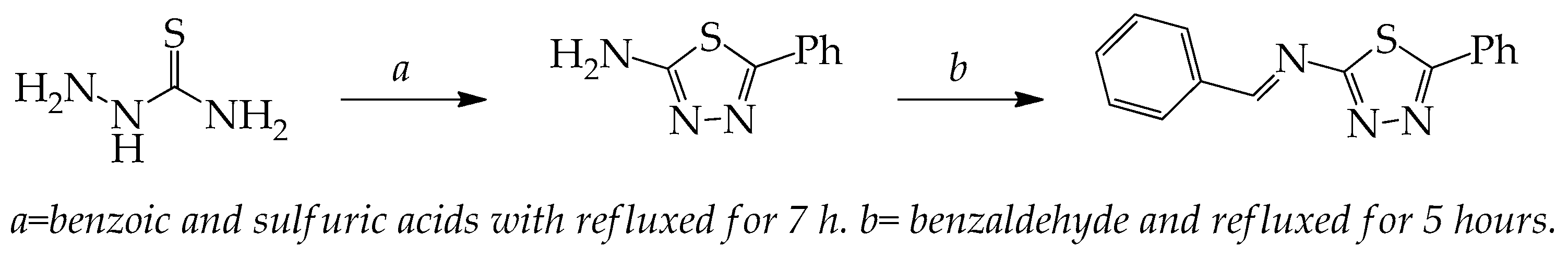Abstract
A New benzylidene derivative namely benzylidene-5-phenyl-1,3,4-thiadiazol-2-amine (BPTA), was successfully synthesized and characterized using Fourier Transform Infrared Spectroscopy, Nuclear Magnetic Resonance and elemental analysis (CHN) techniques. The inhibition efficiency of BPTA on mild steel corrosion in 1.0 N HCl was tested at various temperatures. The methodological work was achieved by gravimetric method complemented with morphological investigation. The concentrations of inhibitor were 0.1, 0.2, 0.3, 0.4 and 0.5 mM at the temperatures 303, 313, 323 and 333 K. The BPTA, molecules as become superior corrosion inhibitor with 92% inhibition efficiency of mild steel coupon in the acidic environment. The inhibition efficiency increased with increasing concentrations of BPTA and the excellent efficiency was performed with the 0.5 mM concentration and followed with 0.4 mM. In acidic environment, the 0.5 and 0.4 mM gave the optimum performance with weight loss technique and scanning electron microscopy analysis. On the other hand, the inhibition efficiency decreased with the increase of temperature. Results of BPTA indicated mixed type inhibitor and the adsorption on the mild steels surface obeys the Langmuir adsorption isotherm. It was found that the BPTA performance depend on the concentration and the solution temperature. Quantum chemical calculations have been done to correlate the electronic characteristics of BPTA with the corrosive inhibitive impact. Experimental and theoretical results are in good agreement.
1. Introduction
Acidic solutions have many applications in industries such as cleaning, pickling, descaling, and acidizing. The uses of HCl in industries was due to its performance action in cleaning in addition to low cost and comparting with other acids. The superior procedure technique to impedance the corrosion of the acidic environment is to use of organic inhibitors. Organic inhibitors having heteroatoms such as P “phosphorus”, S “sulfur” O “oxygen” and N “nitrogen” were mentioned as corrosion inhibitors. The molecules of organic inhibitors were adsorbing on the surface of the mild steel and retard the active site through blocking the reactions occur on the surface of mild steel [1,2]. Organic compounds proved their abilities for corrosion inhibition through adsorption on the metal surface, on the other hand inorganic inhibitors behavior as anodic inhibitor compounds and the iron atoms enclosed in the layer improve their corrosion impedance. The inhibition action depends on parameters such as active groups, structure of the molecule and steric effect [3,4,5]. Following up of the investigations for efficient corrosion inhibitor [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28], this investigation reports the inhibitive effects of new corrosion inhibitor. The synthesized new corrosion inhibitor namely benzylidene-5-phenyl-1,3,4-thiadiazol-2-amine (BPTA), were characterized with FTIR and NMR spectroscopies. The corrosion inhibition behavior on the surface of mild steel in corrosive environment was studied using weight loss techniques and surface investigations using scanning electron microscope. Density functional theory (DFT) were used to corroborate mythological findings.
2. Experimental
2.1. Synthesis of Benzylidene-5-phenyl-1,3,4-thiadiazol-2-amine (BPTA)
Reflux of similar quantities of benzaldehyde and 2-amino-5-phenyl-1,3,4-thiadiazol (0.02 mol) in ethanol for 5 h. Cooling and precipitated filtered and recrystallized from ethanol to obtain benzylidene-5-phenyl-1,3,4-thiadiazol-2-amine (BPTA); M.P. 169.5 °C, Yield 88 %,FT-IR spectrum (υ, cm−1): 1570 (C=N); 1H-NMR (CDCl3-DMSO-d6, δ, ppm): 8.82 (s, 1H, azomethine CH=N), 7.36, 7.55, 7.59, 7.93 and 8.01, (10H aromatic H); 13C-NMR (CDCl3-DMSO-d6, δ, ppm): 173.10 and 174.11 (carbon for thiadiazole ring); 164.31 (CH=N); 128.8, 129.76, 131.26, 134.75, 128.39, 127.38, 132.41, 137.16 (carbon for aromatic rings). Anal. Calculated/found for C15H11N3S: C, 67.90/68.10, H, 4.18/4.14, N, 15.84/15.63.
2.2. Materials and Sample Preparation
The mild steel coupons with the composition (wt.%) of C, 0.21%; Fe, 99.21%; Si, 0.38%; Mn, 0.05%; P, 0.09%; S, 0.05% and Al, 0.01% have been used for corrosion tests and the dimensions of coupons were 3 × 3 cm2. Silicon carbide paper were used to cleaned the coupons and 15 min of sonicated with ethanol. Finally washed with distilled water, acetone and dried. HCl with the concentration if 1.0 M was used as strong acidic environment and was prepared by diluting analytical grade 37% hydrochloric acid with distilled water. The concentration of inhibitors was 0.1, 0.2, 0.3, 0.4 and 0.5 mM.
2.3. Weight Loss Techniques
The gravimetric measurements have been achieved depending on ASTM procedure. The coupons have been immersed in the acidic solution without and with addition of inhibitor at the concentrations of 0.1–0.5 mM. The influence of immersion time 1, 5, 10 and 24 h and the tests were carried out at 303 K and stabilized using water bath. For temperature studies, similar tests were repeated for 5 h, as immersion time. Corrosion rate, Inhibition efficiency, and surface coverage were calculated through Equations (1)–(3).
where CR is the corrosion rate; t is the immersion time, W is the coupon weight loss (in gram), A is the area and d is the density.
where CRO corrosion rate in absence of inhibitor where as CRin corrosion rate in presence of inhibitor.
2.4. Density Functional Theory Calculation
DFT calculations have been used with GAUSSIAN 03W software [29], B3LYP function [30,31] and a 6-31G as basis set [32]. The energies including EHOMO and ELUMO were obtained to calculate further significant parameters such ΔE, η, σ, χ, and ΔN using [33,34] the Equations (4)–(8)
where χFe is the electronegativity of iron and ηinh is the hardness of iron.
It was reported that χFe, was of 7 eV/mol, whereas ηFe was 0 eV/mol [35].
3. Results and Discussion
3.1. Chemistry
The sequences for the reaction synthesis of BPTA starting from 2-amino-5-phenyl-1,3,4-thiadiazol are outlined in Scheme 1. The reaction of benzaldehyde with 2-amino-5-phenyl-1,3,4-thiadiazol afforded benzylidene namely benzylidene-5-phenyl-1,3,4-thiadiazol-2-amine (BPTA) in good yield. The FT-IR spectrum of compound BPTA showed absorption band at 1570 cm−1 (azomethine C=N). The 1H-NMR spectrum exhibited a singlet at δ 8.82 ppm due to the one azomethine proton and this band confirm the formation of target inhibitor. A multiplate due to the aromatic proton at δ 7.36, 7.55, 7.59, 7.93 and 8.01 ppm. The NH2 vibrational band was disappeared for the synthesized inhibitor BPTA that was appeared in the parent compound. The disappearance nitrogen-hydrogen bond BPTA was due to condensation reaction between benzaldehyde with 2-amino-5-phenyl-1,3,4-thiadiazol and reducing H2O to produce the target compound.
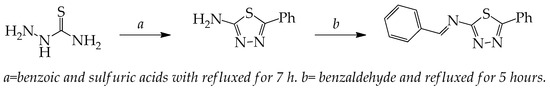
Scheme 1.
The BPTA chemical structure.
3.2. Gravimetric Techniques
3.2.1. Effects of Concentrations
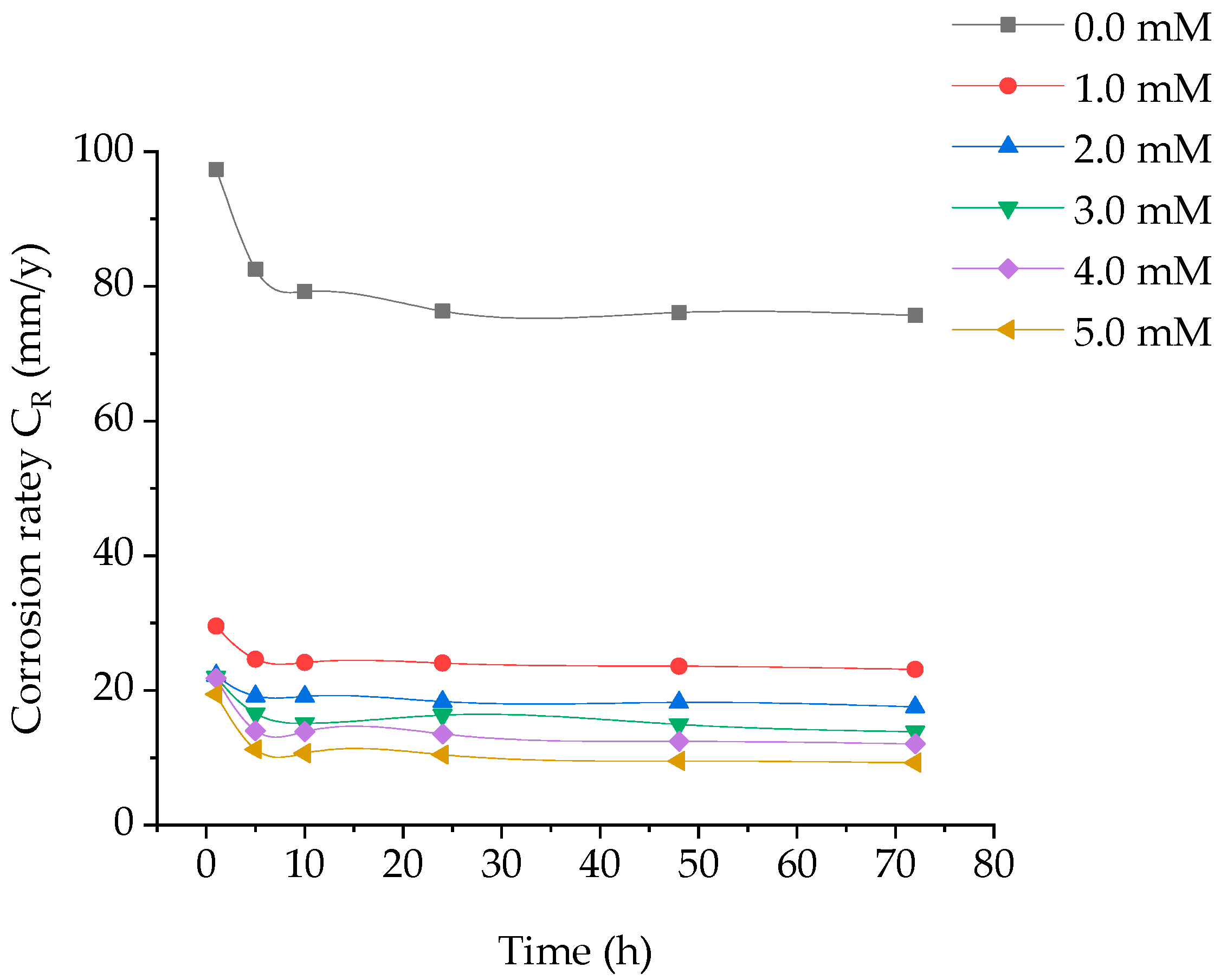
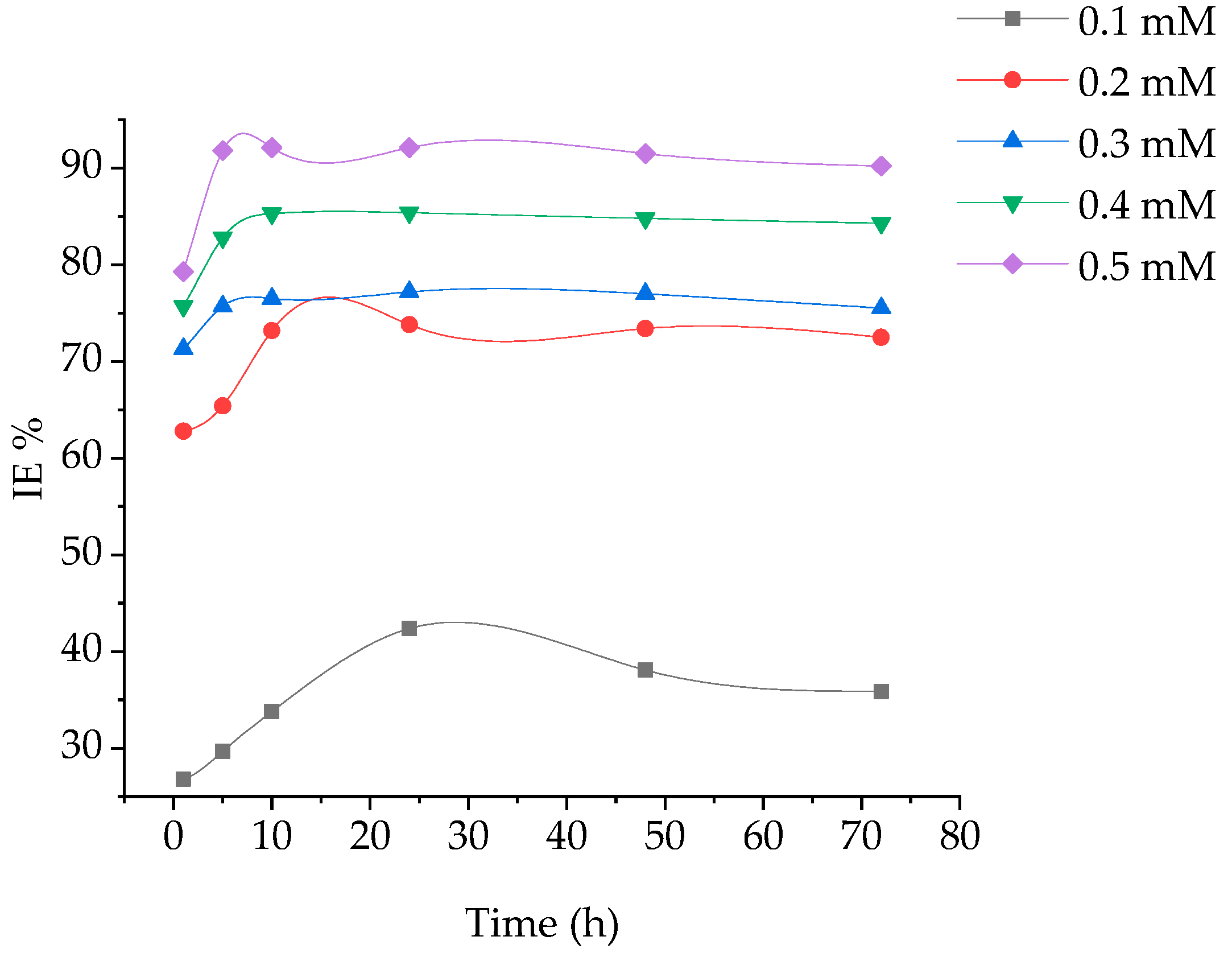
Weight loss of coupons at different time periods, without and with addition of various concentrations of (BPTA) in 1 M hydrochloric acid at 303 K was investigated. The corrosion rate and the inhibition efficiency are displayed in Figure 1 and Figure 2. It is obvious that the reducing CR is related to increase in BPTA concentration which elucidates that furthe BPTA molecules adsorbed on the coupon surface, herewith supplying broader surface coverage.
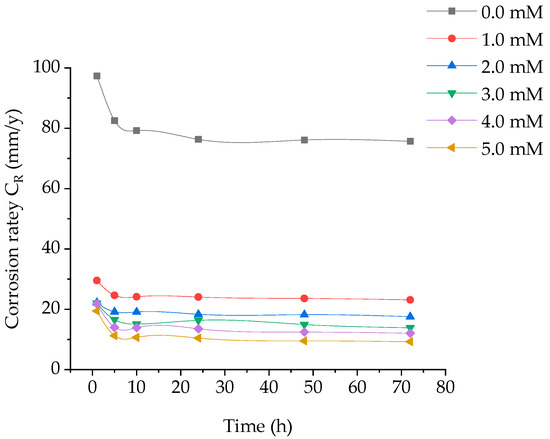
Figure 1.
Effect of concentration of BPTA and time on corrosion rate of tested coupon.
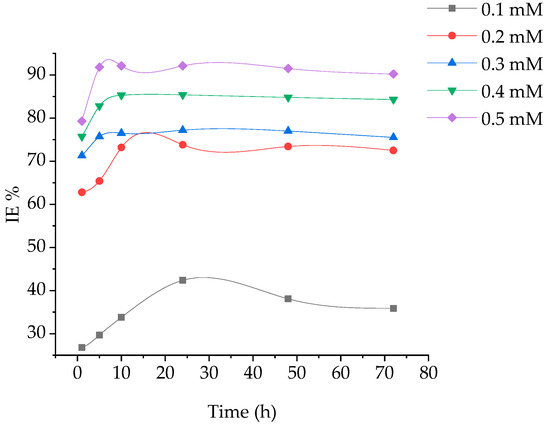
Figure 2.
of immersion time of MS in corrosive environment at various concentrations of BPTA.
The maximum IE of BPTA was 92 that obtained at the optimum concentration of 5 mM at 10 h. The excellent inhibition efficiency was attributed to the heteroatoms (N and S) and aromatic rings (phenyl and 1,3,4-thiadiazole) in the molecular structure of BPTA. These hetero atoms react with the coupon surface through adsorption. The inhibition efficiency increases gradually with increasing time until it reaches its highest level at 5 h and stabilizes up to 10 h due to the rapid absorption of the BPTA molecules on the coupon surface and then start to decrease gradually due to desorption. The corrosion rate increases after 10 h of immersion due to the low inhibitor concentration in the HCl solution leading to the decay of the metal; it is clear that after dissolution BPTA molecules from the metal surface become inactive and therefore not involved in the inhibition.
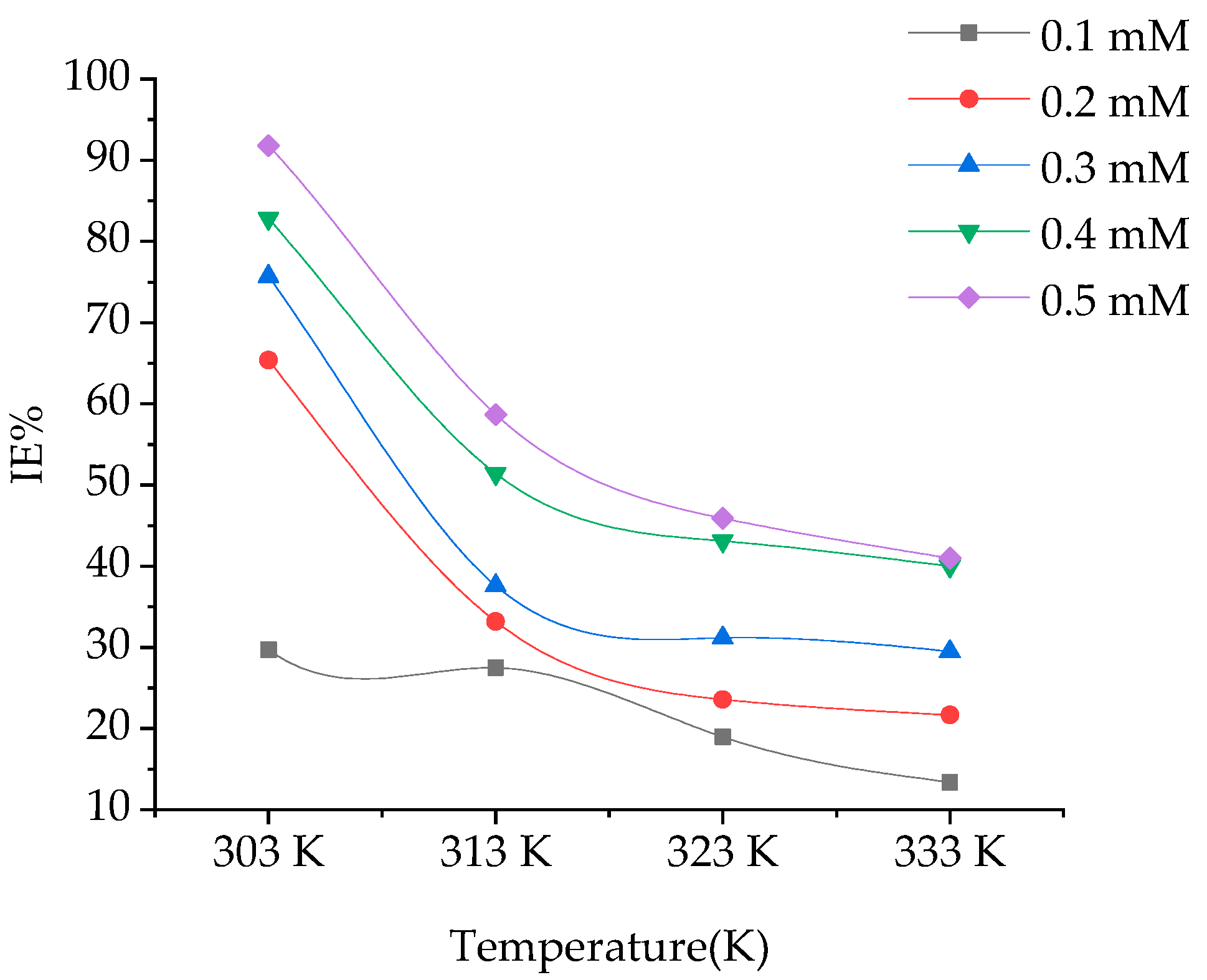
3.2.2. Effect of Temperature
The effect of temperature in the range of 303–333, on Inhibition efficiency in absence and presence of BPTA is shown in Figure 3. From the plot of Inhibition efficiency vs temperature, it is obvious that the Inhibition efficiency decreases with increasing temperature. The inhibition efficiencies obtained at optimum inhibitor (BPTA) concentration were 92, 91 at 303 K and 41 at 333K respectively, in which the minimum and maximum examined temperatures were used in this study.
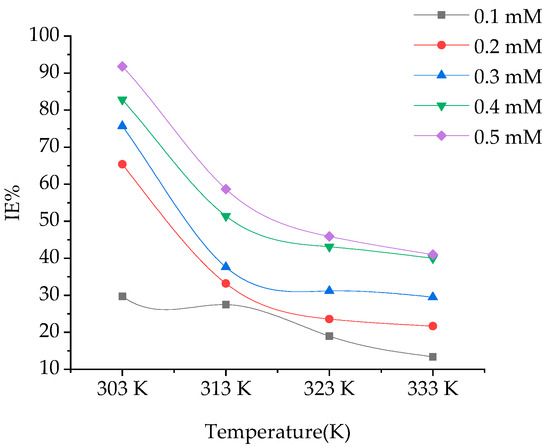
Figure 3.
Effect of temperature on IE of BPTA in 1 M HCl.
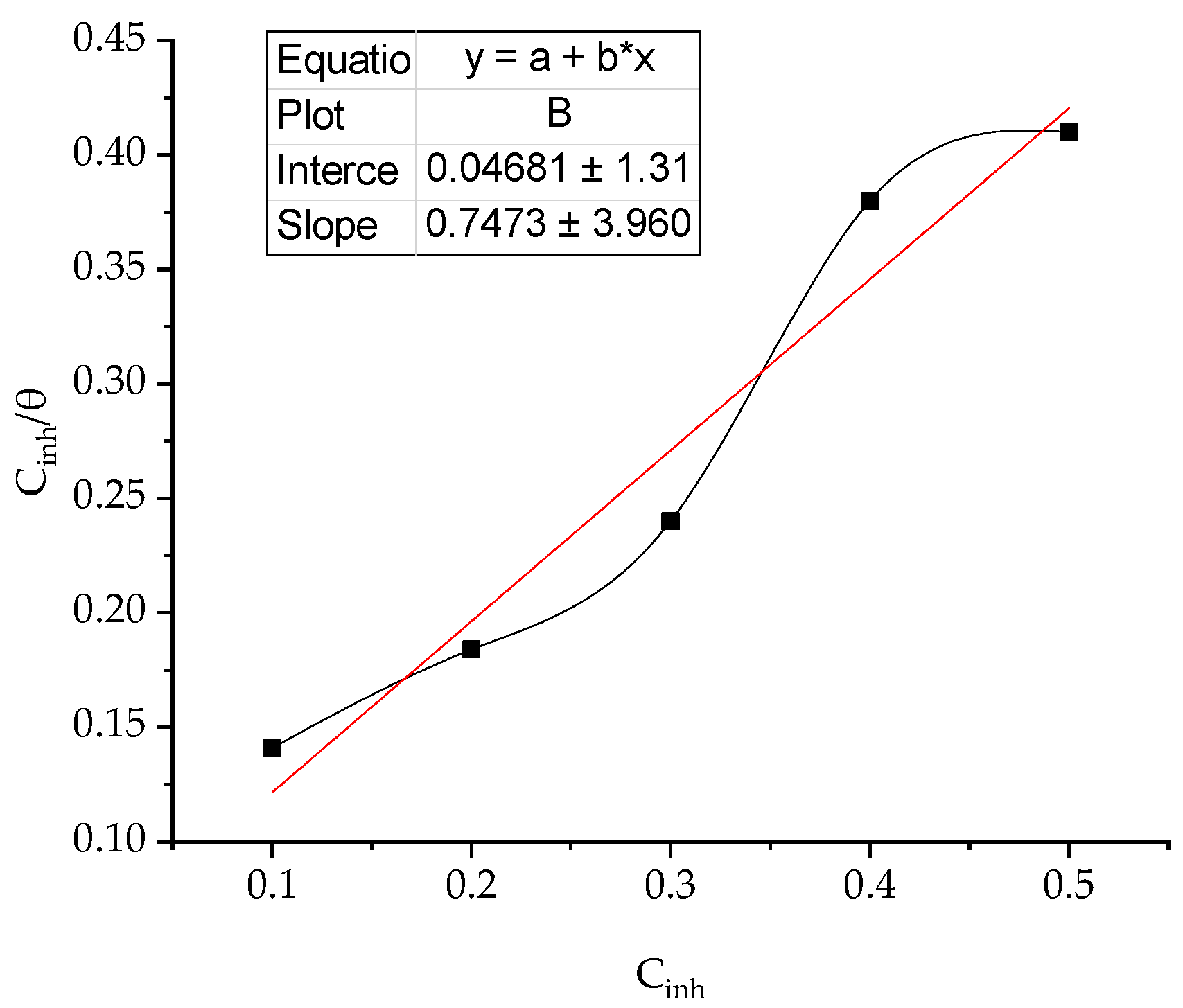
3.3. Adsorption Isotherms
Adsorption isotherm demonstrate information about the mechanism of adsorption of inhibitor on coupon surface [36,37]. The adsorption of BPTA, molecules on to the coupon surface is the initial stage of adsorption mechanism. Thermodynamic parameters were calculate using the Langmuir adsorption model. The surface coverage increases with the BPTA concentration acidic media, due to formation a protected layer of BPTA molecules on to the coupon surface to impedance the corrosion. Equilibrium constant (Kads) can be calculated via equation 9.
where C is the concentration and B is an intercept.
Figure 4 illustrates the plot of the Langmuir isotherm model, which is demonstrated by the presence of correlation coefficients that were close to the unit, implying that the adsorption of the inhibitor molecules on the coupon surface assumes the adsorption of a single layer. Adsorption of inhibitor molecules tested on the coupon surface obeys Langmuir's isotherm.
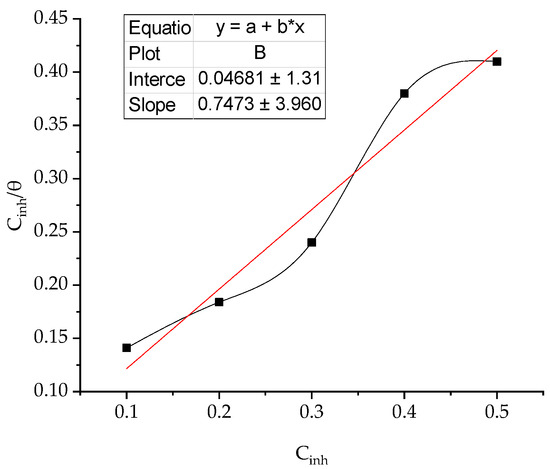
Figure 4.
Langmuir plot for adsorption of corrosion inhibitor molecules on to coupon surface.
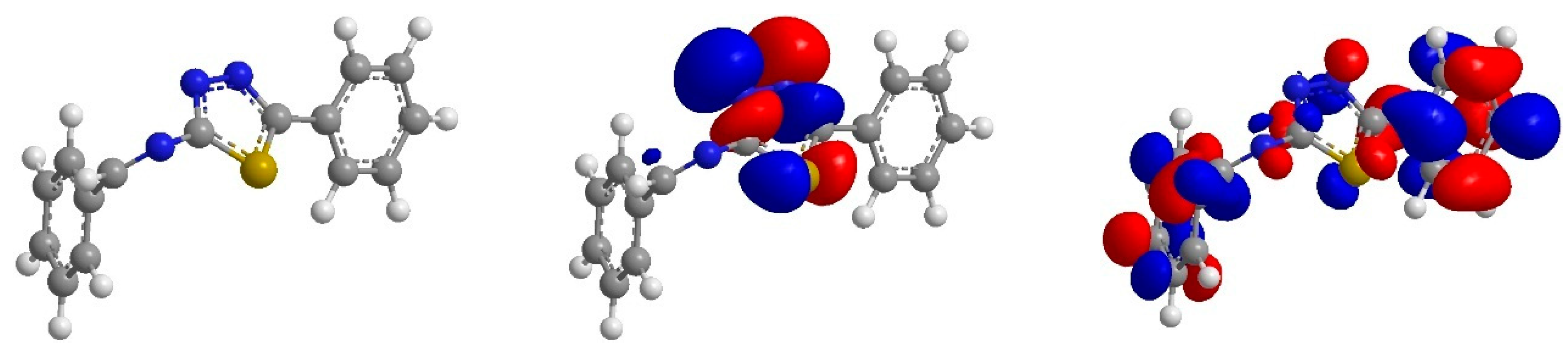
3.4. Quantum Chemistry
From Figure 5 it can be shown that the HOMO orbital BPTA is localize on the thiadiazole ring and azomethine group. These are the regions donating electrons to unoccupied d-orbital of iron atoms, whereas, LUMO orbitals for BPTA are found on all over the molecule. This region accepts electrons from iron atoms. Thus, the HOMO and LUMO orbital analyses indicate that the thiadiazole ring, benzene ring and azomethine groups play an important role as active sites for the interaction of the corrosion inhibitor with coupon surface.
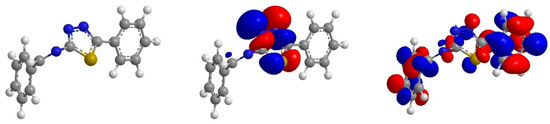
Figure 5.
Optimized structure, HOMO and LUMO orbitals distribution of BPTA.
Table 1 demonstrates the parameters for the BPTA. Values of χ, and η as in Equations (5)–(7), were calculated by using the values of I (-LUMO) and A (-HOMO) obtained from DFT calculation. Using a theoretical χ value of 7 eV/mol and η value of 0 eV/mol for iron atom [38]. ∆N, the fraction of electrons transferred from inhibitor to the iron molecule, was calculated. According to Lukovits’s study [39], if ∆N < 3.6 the inhibition efficiency increased with increasing electron-donating ability at the coupon surface. In this study, BPTA molecules were donate the electrons, and the atoms of iron surface were accepting them. BPTA molecules were bonding to the coupon surface and formed inhibition adsorption layer against corrosive environment.

Table 1.
Characteristics of BPTA.
4. Conclusions
New corrosion inhibitor namely benzylidene-5-phenyl-1,3,4-thiadiazol-2-amine (BPTA), was successfully synthesized and characterized using fourier transform infrared spectroscopy, Nuclear magnetic resonance and elemental analysis (CHN) techniques. The inhibition efficiency for mild steel in hydrochloric acid was evaluated using weight loss and scanning electron microscope techniques. Theoretical investigations were performed to evaluate the adsorption of the inhibitor molecules onto the mild steel surface. Results from the experimental and theoretical considerations are in good agreement confirming that BPTA is an excellent corrosion inhibitor for mild steel in 1 m hydrochloric acid.
Author Contributions
Conceptualization, A.A.A.-A.; methodology, S.B.A.-B. and M.M.H.; software, L.M.S.; validation, J.F.O. and S.B.A.-B.; A.A.A.-A. and L.M.S.; writing—original draft preparation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by University of technology, grant number 2019.
Acknowledgments
The authors gratefully acknowledge University of Technology/Iraq for providing the facilities for the work.
Conflicts of Interest
There are no conflict to declare.
References
- Tian, H.; Li, W.; Liu, A.; Gao, X.; Han, P.; Ding, R.; Yang, C.; Wang, D. Controlled delivery of multi-substituted triazole by metalorganic framework for efficient inhibition of mild steel corrosion in neutral chloride solution. Corros. Sci. 2018, 131, 1–16. [Google Scholar] [CrossRef]
- Dutta, A.; Saha, S.K.; Adhikari, U.; Banerjee, P.; Sukul, D. Effect of substitution on corrosion inhibition properties of 2-(substituted phenyl) benzimidazole derivatives on mild steel in 1 M HCl solution: A combined experimental and theoretical approach. Corros. Sci. 2017, 123, 256–266. [Google Scholar] [CrossRef]
- Jayalakshmi, M.; Muralidharan, V.S. Correlation between structure and inhibition of organic-compounds for acid corrosion of transition-metals. Ind. J. Chem. Tech. 1998, 5, 16–28. [Google Scholar]
- Granese, S.L. Study of the inhibitory action of nitrogencontaining compounds. Corrosion 1998, 44, 322–329. [Google Scholar] [CrossRef]
- Granese, S.L.; Rosales, B.M.; Oviedo, C.; Zebrino, J.O. The inhibition action of heterocyclic nitrogen organic compounds on Fe and steel in HCl media. Corros. Sci. 1992, 33, 1439–1453. [Google Scholar] [CrossRef]
- Junaedi, S.; Kadhum, A.; Al-Amiery, A.; Mohamad, A.; Takriff, M. Synthesis and char-acterization of novel corrosion inhibitor derived from oleic acid: 2-Amino5-Oleyl-1,3,4-Thiadiazol (AOT). Int. J. Electrochem. Sci. 2012, 7, 3543–3554. [Google Scholar]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B.; Junaedi, S. A Novel Hydrazinecarbothioamide as a Potential Corrosion Inhibitor for Mild Steel in HCl. Materials 2013, 6, 1420–1431. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kadhum, A.; Mohamad, A.; Musa, A.; Li, C. Electrochemical study on newly synthesized chlorocurcumin as an inhibitor for mild steel corrosion in hy-drochloric acid. Materials 2013, 6, 5466–5477. [Google Scholar] [CrossRef]
- Salman, T.; Al-Amiery, A.; Shaker, L.; Kadhum, A.; Takriff, S. A study on the inhibition of mild steel corrosion in hydrochloric acid environment by 4-methyl-2-(pyridin-3-yl)thiazole-5-carbohydrazide. Int. J. Corros. Scale Inhib. 2019, 8, 1035–1059. [Google Scholar]
- Kadhum, A.A.H.; Mohamad, A.B.; Hammed, L.A.; Al-Amiery, A.A.; San, N.H.; Musa, A.Y. Inhibition of Mild Steel Corrosion in Hydrochloric Acid Solution by New Coumarin. Materials 2014, 7, 4335–4348. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Kadihum, A.; Mohamad, A.B.; How, C.K.; Junaedi, S. Inhibition of Mild Steel Corrosion in Sulfuric Acid Solution by New Schiff Base. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel Corrosion Inhibitor for Mild Steel in HCl. Materials 2014, 7, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.; Kadhum, A.; Al-Amiery, A.; Ying, L.; Musa, A. Synergistic of a coumarin derivative with potassium iodide on the corrosion inhibition of aluminum alloy in 1.0M H2SO4. Met. Mater. Int. 2014, 20, 459–467. [Google Scholar] [CrossRef]
- Obayes, R.; Al-Amiery, A.; Alwan, G.; Alobaidy, A.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Quantum chemical assessment of benzimidazole derivatives as corrosion Inhibitors. Chem. Cent. J. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.H.; Mohamad, A.B. New Coumarin Derivative as an Eco-Friendly Inhibitor of Corrosion of Mild Steel in Acid Medium. Molecules 2015, 20, 366–383. [Google Scholar] [CrossRef]
- Yousif, E.; Win, Y.; Al-Hamadani, A.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Furosemi as an environmental-friendly inhibitor of corrosion of zinc metal in acid medium experimental and theoretical studies. Int. J. Electrochem. Sci. 2015, 10, 1708–1718. [Google Scholar]
- Rubaye, A.; Abdulwahid, A.; Al-Baghdadi, S.; Al-Amiery, A.; Kadhum, A.; Mohamad, A. Cheery sticks plant extract as a green corrosion inhibitor complemented with LC-EIS/ MS spectroscopy. Int. J. Electrochem. Sci. 2015, 10, 8200–8209. [Google Scholar]
- Al-Obaidy, A.; Kadhum, A.; Al-Baghdadi, S.; Al-Amiery, A.; Kadhum, A.; Yousif, E. Eco-friendly corrosion inhibitor: Experimental studies on the corrosion inhibition performance of creatinine for mild steel in HCl complemented with quantum chemical calculations. Int. J. Electrochem. Sci. 2015, 10, 3961–12972. [Google Scholar]
- Al-Baghdadi1, S.B.; Noori, F.T.M.; Ahmed, W.K.; Al-Amiery, A.A. Thiadiazole as a Potential Corrosion Inhibitor for Mild Steel in 1 M HCl. J. Adv. Electrochem. 2016, 2, 67–69. [Google Scholar]
- Al-Amiery, A.A.; Kassim, F.A.B.; Kadhum, A.A.H.; Mohamad, A.B. Synthesis and characterization of a novel eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric acid. Sci. Rep. 2016, 6, 19890. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Okbi, A.K.; Jamil, D.M.; Qussay, A.; Al-Amiery, A.A.; Gaaz, T.S. Experimental and theoretical studies of benzoxazines corrosion inhibitors. Results Phys. 2017, 7, 4013–4019. [Google Scholar] [CrossRef]
- Obayes, R.; Al-Amiery, A.; Alwan, G.; Abdullah, T.; Kadhum, A.; Mohamad, A. Sulphonamides as corrosion inhibitor: Experimental and DFT studies. J. Mol. Struct. 2017, 1138, 27–34. [Google Scholar] [CrossRef]
- Al-Baghdadi, S.B.; Hashim, F.G.; Salam, A.Q.; Abed, T.K.; Gaaz, T.S.; Al-Amiery, A.A.; Kadhum, A.H.; Reda, K.S.; Ahmed, W.K. Synthesis and corrosion inhibition application of NATN on mild steel surface in acidic media complemented with DFT studies. Results Phys. 2018, 8, 1178–1184. [Google Scholar] [CrossRef]
- Habeeb, H.J.; Luaibi, H.M.; Dakhil, R.M.; Kadhum, A.H.; Al-Amiery, A.A.; Gaaz, T.S. Development of new corrosion inhibitor tested on mild steel supported by electrochemical study. Results Phys. 2018, 8, 1260–1267. [Google Scholar] [CrossRef]
- Al-Azawi, K.F.; Mohammed, I.M.; Al-Baghdadi, S.B.; Salman, T.A.; Issa, H.A.; Al-Amiery, A.A.; Gaaz, T.S.; Kadhum, A.A.H. Experimental and quantum chemical simulations on the corrosion inhibition of mild steel by 3-((5-(3,5- dinitrophenyl)-1,3,4-thiadiazol-2-yl)imino)indolin-2-one. Results Phys. 2018, 9, 278–283. [Google Scholar] [CrossRef]
- Jamil, D.M.; Al-Okbi, A.K.; Al-Baghdadi, S.B.; Al-Amiery, A.A.; Kadhim, A.; Gaaz, T.S. Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem. Cent. J. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Ahmed, M.; Al-Amiery, A.; Al-Majedy, Y.; Kadhum, A.; Mohamad, A.; Gaaz, T. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 8, 728–733. [Google Scholar] [CrossRef]
- Salman, T.; Zinad, D.; Jaber, S.; Al-Ghezi, M.; Mahal, A.; Takrif, M.; Al-Amiery, A. Effect of 1,3,4-Thiadiazole Scafold on the Corrosion Inhibition of Mild Steel in Acidic Medium: An Experimental and Computational Study. J. Bio-Tribo-Corros. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Güngör, Ö.; Gürkan, P. Synthesis and characterization of higher amino acid Schiff bases, as monosodium salts and neutral forms. Investigation of the intramolecular hydrogen bonding in all Schiff bases, antibacterial and antifungal activities of neutral forms. J. Mol. Struct. 2014, 1074, 62–70. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef]
- Şafak, S.; Duran, B.; Yurt, A.; Türkoĝlu, G. Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros. Sci. 2012, 54, 251–259. [Google Scholar] [CrossRef]
- Gaussian 03, Revision C.02; Gaussian, Inc.: Pittsburgh, PA, USA, 2003.
- Ahamad, I.; Prasad, R.; Quraishi, M.A. Experimental and quantum chemical characterization of the adsorption of some Schiff base compounds of phthaloyl thiocarbohydrazide on the mild steel in acid solutions. Mater. Chem. Phys. 2010, 124, 1155–1165. [Google Scholar] [CrossRef]
- Lashgari, M.; Arshadi, M.R.; Parsafar, G.A. A simple and fast method for comparison of corrosion inhibition powers between pairs of pyridine derivative molecules. Corrosion 2005, 61, 778–783. [Google Scholar] [CrossRef]
- Wang, J.; Lian, Z.; Wang, H.; Jin, X.; Liu, Y. Synthesis and antimicrobial activity of Schiff base of chitosan and acylated chitosan. J. Appl. Polym. Sci. 2012, 123, 3242–3247. [Google Scholar] [CrossRef]
- Sabirneeza, A.A.F. A novel water-soluble, conducting polymer composite for mild steel acid corrosion inhibition. J. Appl. Polym. Sci. 2016, 127, 3084–3092. [Google Scholar] [CrossRef]
- Biswas, A.; Pal, S.; Udayabhanu, G. Experimental and theoretical studies of xanthan gum and its graft co-polymer as corrosion inhibitor for mild steel in 15% HCl. Appl. Surface Sci. 2015, 353, 173–183. [Google Scholar] [CrossRef]
- Sastri, V.S.; Perumareddi, J.R. Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 1997, 53, 617–622. [Google Scholar] [CrossRef]
- Lukovits, I.; Kalm’an, E.; Zucchi, F. Corrosion inhibitors—Correlation between electronic structure and efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).