Abstract
In this study, a series of nine 3-hydroxynaphthalene-2-carboxanilides, disubstituted on the anilide ring by fluorine, chlorine and bromine in various positions, was prepared by microwave-assisted synthesis and characterized. The compounds were tested for their activity related to the inhibition of photosynthetic electron transport (PET) in spinach (Spinacia oleracea L.) chloroplasts. The PET-inhibiting activity of the compounds was within a wide range, but rather moderate; the highest activity within the series of the compounds was observed for N-(3,5-difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (IC50 = 9.8 µM). The compounds were found to inhibit PET in photosystem II.
1. Introduction
The presence of an amide (–CONH–) group in the structure of compounds enables interaction with various enzymatic systems [1,2,3,4] and is also characteristic for a number of herbicides acting as inhibitors of photosynthesis [5,6,7,8,9]. Although at present approximately 20 modes of action of herbicides are known [10], over 50% of commercially available herbicides act by reversible binding to photosystem II (PS II) [11], and due to this interaction, the photosynthetic electron transport (PET) is interrupted [12,13,14,15,16].
A series of 3-hydroxy-N-arylnaphthalene-2-carboxanilides monosubstituted on the anilide ring was prepared and published recently [17,18]. As the observed PET inhibition by these compounds in spinach chloroplasts (Spinacia oleracea L.) was moderate and inspired by the high PET-inhibiting activity of halogenated 1-hydroxynaphthalene-2-carboxanilides [19], a new series of 3-hydroxy-N-arylnaphthalene-2-carboxanilides disubstituted by fluorine, chlorine, or bromine on the anilide nucleus was designed to increase PET activity. Thus, this short contribution is a follow-up work to the previous papers [9,17,19,20,21,22,23,24,25,26,27] aimed at the investigation of the PET-inhibiting activity of (aza)naphthalenecarboxamides. The structure-activity relationships are briefly discussed.
2. Results and Discussion
All the studied compounds were prepared according to Scheme 1 as described previously by Kos et al. [17]. The condensation of 3-hydroxynaphthalene-2-carboxylic acid with appropriate substituted anilines using phosphorus trichloride in dry chlorobenzene under microwave conditions gave a series of target 3-hydroxy-N-arylnaphthalene-2-carboxanilides 1–9, see Table 1
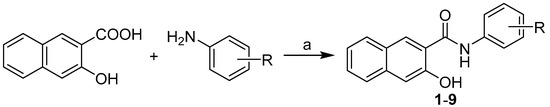
Scheme 1.
Synthesis of 3-hydroxy-N-arylnaphthalene-2-carboxanilides 1–9. Reagents and conditions: (a) PCl3, chlorobenzene, MW, 15 min. [17].

Table 1.
Structure of 3-hydroxynaphthalene-2-carboxanilides 1–9, calculated values of log P of compounds, electronic σ parameters of anilide (Ar) substituents and IC50 [µM] values related to PET inhibition in spinach chloroplasts of tested compounds in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard.
All the predicted molecular descriptors (lipophilicity and electronic σ parameters of individual anilides) were calculated using the ACD/Percepta ver. 2012 program (Advanced Chemistry Development, Toronto, ON, Canada), see Table 1. The lipophilicity of compounds 1–9, expressed as log P values, ranged from 4.53 (compounds 2, R = 2,6-F) to 6.01 (compound 7, R = 3,5-Cl). In general, it can be stated that the lipophilicity is rather high, but for agrochemicals, higher lipophilicity (log P ≤ 5) is recommended [28], because they have to permeate through the hydrophobic cuticle of the plants. Derivatives substituted with the fluoro moiety showed the lowest lipophilicity, compounds substituted by chlorine and bromine atoms possessed approximately similar log P values that were higher than those of fluoro derivatives 1–3. Compounds substituted by fluorine showed the order of log P values as follows: 2 (R = 2,6-F) < 1 (R = 2,5-F) < 3 (R = 3,5-F). The order of log P values of the chlorinated derivatives was as follows: 5 (R = 2,6-Cl) < 4 (R = 2,5-Cl) < 6 (R = 3,4-Cl) < 7 (R = 3,5-Cl). Compound 9 (R = 2,5-Br) was less lipophilic than compound 8 (R = 2,4-Br).
The PET-inhibiting activity was expressed by negative logarithm of IC50 value (compound concentration in µM causing 50% inhibition of PET). The evaluated dihalogenated 3-hydroxy- naphthalene-2-carboxanilides showed a wide range of PET-inhibiting activity related to PET inhibition in spinach (Spinacia oleracea L.) chloroplasts with IC50 values ranging from 9.8 to 1123 µM, see Table 1, while in general, the PET inhibition was rather moderate. N-(3,5-Difluorophenyl)- 3-hydroxynaphthalene-2-carboxamide (3) showed the highest PET-inhibiting activity (IC50 = 9.8 µM) within the whole investigated series, while positional isomer N-(2,5-difluorophenyl)-3-hydroxy- naphthalene-2-carboxamide (1) was completely inactive (IC50 = 1123 µM).
The dependences of the PET-inhibiting activity expressed as log(1/IC50 [M]) of compounds 1–9 in spinach chloroplasts on lipophilicity and electronic σ(Ar) properties of the whole anilide substituents are shown in Figure 1. Due to small number of fluorine and bromine derivatives, it is impossible to formulate structure-activity relationships. On the other hand, it is evident that PET-inhibiting activity increased linearly with an increase of the log P values within a series of chlorinated derivatives 4–7 (correlation coefficient r = 0.9628, n = 4), see Figure 1A, while decreasing linearly with an increase of the electron-withdrawing properties of the anilide ring (correlation factor r = 0.9284, n = 4), see Figure 1B, which influences the electron density on the CONH moiety, resulting in the ability of the amide bond to interact with enzymatic systems. A dependence of PET inhibition on the electron-withdrawing effect of substituents in individual series of many PET inhibitors was observed [9,17,19,20,21,22,24,25]. The disubstitution of the anilide ring on positions C(3,5)′, i.e., on both meta positions, is the most advantageous for high PET inhibition.
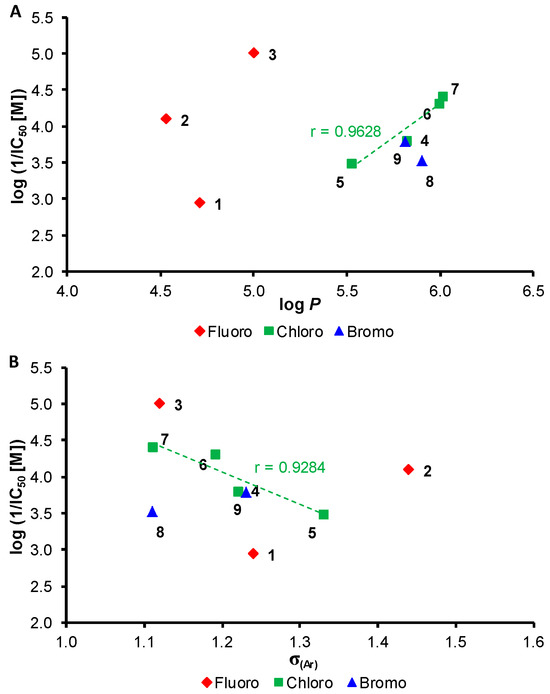
Figure 1.
Dependence of PET-inhibiting activity log(1/IC50 [M]) of compounds 1–9 in spinach chloroplasts on lipophilicity expressed as log P (A) and electronic σ(Ar) parameters of anilide substituents (B).
Thus, it can be concluded that although this investigated series of compounds did not show high activity, such as analogous 1-hydroxynaphthalene-2-carboxanilides published by Gonec et al. [19], these new dihalogenated anilides of 3-hydroxynaphtalene-2-carboxylic acid were significantly more potent than their monosubstituted parent compounds [17].
Based on close structural similarity to other salicylanilides or hydroxyl(aza)naphthanilides, the same mechanism of action of the investigated compounds can be assumed. The site of inhibitory action of the halogenated 3-hydroxynaphthalene-2-carboxanilides is situated on the acceptor side of PS II, at the section between P680 (primary donor of PS II) and plastoquinone QB [8,9,17,19,20,21,22,23,24,25,26,27,29,30,31,32,33].
3. Experimental
3.1. General
All reagents were purchased from Merck (Sigma-Aldrich, St. Louis, MO, USA) and Alfa (Alfa-Aesar, Ward Hill, MA, USA). Reactions were performed using a CEM Discover SP microwave reactor (CEM, Matthews, NC, USA). The melting points were determined on a Kofler hot-plate apparatus HMK (Franz Kustner Nacht KG, Dresden, Germany) and are uncorrected. Infrared (IR) spectra were recorded on a Smart MIRacle™ ATR ZnSe for Nicolet™ Impact 410 Fourier-transform IR spectrometer (Thermo Scientific, West Palm Beach, FL, USA). The spectra were obtained by the accumulation of 256 scans with 2 cm−1 resolution in the region of 4000–650 cm−1. All 1H- and 13C-NMR spectra were recorded on an Agilent VNMRS 600 MHz system (600 MHz for 1H and 150 MHz for 13C, Agilent Technologies, Santa Clara, CA, USA) in dimethyl sulfoxide-d6 (DMSO-d6). 1H and 13C chemical shifts (δ) are reported in ppm. High-resolution mass spectra were measured using a high-performance liquid chromatograph Dionex UltiMate® 3000 (Thermo Scientific, West Palm Beach, FL, USA) coupled with an LTQ Orbitrap XLTM Hybrid Ion Trap-Orbitrap Fourier Transform Mass Spectrometer (Thermo Scientific) equipped with a HESI II (heated electrospray ionization) source in the positive mode.
3.2. Synthesis
General procedure for synthesis of carboxamide derivatives 1–9: 3-Hydroxynaphtalene-2-carboxylic acid (1.0 g, 5.3 mM) was suspended in dry chlorobenzene (30 mL) at ambient temperature and phosphorus trichloride (0.23 mL, 2.7 mM, 0.5 eq.), and the corresponding substituted aniline (5.3 mM, 1 eq.) was added dropwise. The reaction mixture was transferred to the microwave reactor, where the synthesis was performed (1st phase: 10 min, 100 °C, 100 W; 2nd phase: 15 min, 120 °C, 500 W; 3rd phase: 20 min, 130 °C, 500 W). Then the mixture was cooled to 60 °C, and then the solvent was removed to dryness under reduced pressure. The residue was washed with hydrochloride acid and water. The crude product was recrystallized from EtOH. Studied compounds 1–9 are presented in Table 1.
- N-(2,5-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (1). Yield 53%; Mp 264 °C; IR (cm−1): 3057, 1652, 1630, 1598, 1561, 1516, 1489, 1444, 1391, 1360, 1346, 1285, 1272, 1245, 1222, 1184, 1145, 1064, 1015, 972, 949, 908, 889, 862, 833, 807, 795, 765, 736, 716; 1H-NMR (DMSO-d6) δ: 7.00–7.04 (m, 1H), 7.37 (s, 1H), 7.38 (ddd, J = 1.2, 6.8, 8.2 Hz, 1H), 7.41 (ddd, J = 5.1, 9.1, 10.6 Hz, 1H), 7.54 (ddd, J = 1.1, 6.8, 8.3 Hz, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.30 (ddd, J = 3.2, 6.2, 10.3 Hz, 1H), 8.68 (s, 1H), 11.11 (s, 1H), 11.93 (s, 1H); 13C-NMR (DMSO-d6), δ: 108.7 (d, J = 29.9 Hz), 110.4 (dd, J = 8.0, 24.3 Hz), 110.9, 116.1 (dd, J = 10.0, 22.1 Hz), 120.4, 124.1, 125.7, 127.2, 127.6 (t, J = 12.4 Hz), 128.6, 129.1, 132.7, 136.1, 148.8 (dd, J = 2.4, 239.1 Hz), 152.5, 157.9 (dd, J = 1.7, 238.4 Hz), 163.6; HR-MS: for C17H12F2NO2 [M+H]+ calculated 300.0830 m/z, found 300.0832 m/z.
- N-(2,6-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (2). Yield 78%; Mp 194–198 °C; IR (cm−1): 3357, 1662, 1628, 1596, 1511, 1466, 1304, 1285, 1213, 1147, 1134, 1009, 899, 830, 788, 778, 747; 1H-NMR (DMSO-d6), δ: 11.48 (s, 1H), 10.41 (s, 1H), 8.61 (s, 1H), 7.94 (d, 1H, J = 8.1 Hz), 7.78 (d, 1H, J = 8.1 Hz), 7.54 (t, 1H, J = 7.0 Hz), 7.33–7.44 (m, 4H), 7.25 (t, 1H, J = 7.9 Hz); 13C-NMR (DMSO-d6), δ: 166.00, 157.94 (dd, J = 247.3, 5.3 Hz), 154.05, 136.17, 131.21, 128.90, 128.68 (t, J = 19.8 Hz), 128.51, 126.81, 125.94, 125.82, 123.91, 119.45, 114.23 (t, J = 16.7 Hz), 111.95 (m); HR-MS: [M-H]+ calculated 298.0674 m/z, found 298.0681 m/z.
- N-(3,5-Difluorophenyl)-3-hydroxynaphthalene-2-carboxamide (3). Yield 79%; Mp 273–276 °C; IR (cm−1): 3103, 1645, 1626, 1608, 1574, 1564, 1479, 1439, 1345, 1308, 1268, 1225, 1208, 1169, 1118, 999, 989, 855, 829, 767, 740; 1H-NMR (DMSO-d6), δ: 11.05 (s, 1H), 10.80 (s, 1H), 8.38 (s, 1H), 7.93 (d, 1H, J = 8.0 Hz), 7.76 (d, 1H, J = 8.4 Hz), 7.51–7.62 (m, 3H), 7.38 (t, 1H, J = 7.5 Hz), 7.34 (s, 1H), 6.99 (t, 1H, J = 8.0 Hz); 13C-NMR (DMSO-d6), δ: 165.83, 162.43 (dd, J = 241.3, 15.2 Hz), 153.04, 141.19 (t, J = 13.7 Hz), 135.71, 130.55, 128.67, 128.16, 126.87, 125.81, 123.80, 122.80, 110.45, 103.01 (m), 98.93 (t, J = 25.8 Hz); HR-MS: [M-H]+ calculated 298.0674 m/z, found 298.0685 m/z.
- N-(2,5-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (4). Yield 72%; Mp 252 °C; IR (cm−1): 3187, 1637, 1623, 1590, 1580, 1519, 1470, 1455, 1404, 1359, 1347, 1313, 1265, 1204, 1174, 1140, 1090, 1073, 1047, 1017, 979, 960, 916, 867, 846, 808, 769, 750, 707; 1H-NMR (DMSO-d6) δ: 7.25 (dd, J = 2.6, 8.6 Hz, 1H), 7.37 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.38 (s, 1H), 7.53 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.60 (d, J = 8.6 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.67 (d, J = 2.5 Hz, 1H), 8.71 (s, 1H), 11.28 (s, 1H), 12.03 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.9, 120.3, 121.35, 121.39, 124.0, 124.5, 125.7, 127.2, 128.7, 129.1, 130.6, 132.1, 133.0, 136.1, 136.5, 152.4, 163.5; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0243 m/z.
- N-(2,6-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (5). Yield 73%; Mp 283–284 °C; IR (cm−1): 3209, 1623, 1614, 1570, 1515, 1463, 1450, 1433, 1405, 1328, 1236, 1205, 1155, 970, 912, 818, 793, 783, 771, 745, 704, 679; 1H-NMR (DMSO-d6) δ: 11.37 (s, 1H), 10.45 (s, 1H), 8.60 (s, 1H), 7.95 (d, J = 8.2 Hz, 1H), 7.78 (d, J = 8.2 Hz, 1H), 7.65 (d, J = 8.2 Hz, 2H), 7.51 (ddd, J = 7.8, 6.9, 1.0 Hz, 1H), 7.47 (t, J = 8.2 Hz, 1H), 7.36 (ddd, J = 8.2, 6.9, 0.9 Hz, 1H), 3.35 (s, 1H); 13C-NMR (DMSO-d6), δ: 165.79, 153.63, 136.00, 134.07, 133.27, 131.08, 129.34, 128.86, 128.78, 128.23, 126.83, 125.90, 125.56, 123.86, 120.36; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0238 m/z.
- N-(3,4-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (6). Yield 76%; Mp 278–279 °C; IR (cm−1): 3080, 1622, 1605, 1589, 1566, 1545, 1474, 1449, 1396, 1377, 1358, 1344, 1240, 1207, 1172, 1134, 1073, 1024, 955, 925, 898, 852, 837, 826, 771, 748, 680; 1H-NMR (DMSO-d6) δ: 11.09 (s, 1H), 10.75 (s, 1H), 8.41 (s, 1H), 8.18 (d, J = 2.3 Hz, 1H), 7.93 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.72 (dd, J = 8.9, 12.3 Hz, 1H), 7.64 (d, J = 8.7 Hz, 1H), 7.51 (ddd, J = 7.8, 6.9, 0.9 Hz, 1H), 7.36 (d, J = 8.2, 6.9, 0.9 Hz, 1H), 7.33 (s, 1H); 13C-NMR (DMSO-d6), δ: 165.81, 153.22, 138.77, 135.73, 131.03, 130.70, 130.51,128.67, 128.16, 126.85, 125.82, 125.34, 123.81, 122.54, 121.43, 120.29, 110.47; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0242 m/z.
- N-(3,5-Dichlorophenyl)-3-hydroxynaphthalene-2-carboxamide (7). Yield 70%; Mp 252 °C; IR (cm−1): 3087, 1646, 1630, 1583, 1547, 1447, 1409, 1396, 1375, 1345, 1329, 1269, 1254, 1204, 1172, 1147, 1115, 1092, 1072, 1017, 993, 938, 917, 905, 867, 861, 836, 802, 788, 764, 741, 723, 688; 1H-NMR (DMSO-d6) δ: 7.34 (s, 1H), 7.34 (t, J = 1.7 Hz, 1H), 7.36 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.51 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.89 (d, J = 1.7 Hz, 2H), 7.92 (d, J = 8.2 Hz, 1H), 8.39 (s, 1H), 10.75 (s, 1H), 11.04 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.5, 118.3, 122.6, 123.0, 123.8, 125.8, 126.8, 128.2, 128.7, 130.5, 134.1, 135.7, 141.0, 153.1, 165.9; HR-MS: for C17H12Cl2NO2 [M+H]+ calculated 332.0240 m/z, found 332.0244 m/z.
- N-(2,4-Dibromophenyl)-3-hydroxynaphthalene-2-carboxamide (8). Yield 45%; Mp 241 °C; IR (cm−1): 3221, 1641, 1625, 1603, 1575, 1524, 1462, 1448, 1398, 1363, 1345, 1321, 1290, 1240, 1206, 1175, 1146, 1081, 1035, 951, 913, 878, 867, 846, 825, 791, 767, 737, 688; 1H-NMR (DMSO-d6) δ: 7.37 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.38 (s, 1H), 7.53 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.66 (dd, J = 2.2, 8.8 Hz, 1H), 7.78 (d, J = 8.3 Hz, 1H), 7.97 (d, J = 2.2 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 8.42 (d, J = 8.8 Hz, 1H), 8.70 (s, 1H), 11.07 (s, 1H), 11.97 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.8, 114.9, 116.3, 120.4, 124.0, 124.4, 125.7, 127.2, 128.6, 129.1, 131.3, 132.8, 134.4, 136.1, 136.2, 152.6, 163.6; HR-MS: for C17H12O2NBr2 [M+H]+ calculated 419. 9229 m/z, found 419.9237 m/z.
- N-(2,5-Dibromophenyl)-3-hydroxynaphthalene-2-carboxamide (9). Yield 31%; Mp 233 °C; IR (cm−1): 3190, 1636, 1622, 1597, 1568, 1506, 1447, 1393, 1360, 1344, 1250, 1192, 1174, 1147, 1080, 1069, 1029, 962, 915, 902, 868, 848, 796, 770, 750, 736; 1H-NMR (DMSO-d6) δ: 7.32 (dd, J = 2.4, 8.5 Hz, 1H), 7.38 (ddd, J = 1.1, 6.8, 8.2 Hz, 1H), 7.39 (s, 1H), 7.54 (ddd, J = 1.2, 6.8, 8.3 Hz, 1H), 7.69 (d, J = 8.5 Hz, 1H), 7.79 (d, J = 8.3 Hz, 1H), 8.00 (d, J = 8.2 Hz, 1H), 8.71 (s, 1H), 8.74 (d, J = 2.3 Hz, 1H), 11.14 (s, NH, 1H), 12.02 (s, 1H); 13C-NMR (DMSO-d6), δ: 110.8, 112.7, 120.3, 120.9, 124.0, 125.0, 125.7, 127.2, 128.1, 128.7, 129.1, 133.0, 134.2, 136.2, 138.1, 152.5, 163.6; HR-MS: for C17H12O2NBr2 [M+H]+ calculated 419.9229 m/z, found 419.9239 m/z.
3.3. Study of Photosynthetic Electron Transport (PET) Inhibition in Spinach Chloroplasts
Chloroplasts were prepared from spinach (Spinacia oleracea L.) according to Kralova et al. [34]. The inhibition of photosynthetic electron transport (PET) in spinach chloroplasts was determined spectrophotometrically (Genesys 6, Thermo Scientific), using an artificial electron acceptor 2,6-dichlorophenol-indophenol (DCIPP) according to Kralova et al. [34], and the rate of photosynthetic electron transport was monitored as a photoreduction of DCPIP. The measurements were carried out in phosphate buffer (0.02 M, pH 7.2) containing sucrose (0.4 M), MgCl2 (0.005 M), and NaCl (0.015 M). The chlorophyll content was 30 mg/L in these experiments, and the samples were irradiated (~100 W/m2 with 10 cm distance) with a halogen lamp (250 W) using a 4 cm water filter to prevent warming of the samples (suspension temperature 22 °C). The studied compounds were dissolved in DMSO due to their limited water solubility. The applied DMSO concentration (up to 4%) did not affect the photochemical activity in spinach chloroplasts. The inhibitory efficiency of the studied compounds was expressed by IC50 values, i.e., by molar concentration of the compounds causing a 50% decrease in the oxygen evolution rate relative to the untreated control. The comparable IC50 value for the selective herbicide 3-(3,4-dichlorophenyl)-1,1-dimethylurea, DCMU (Diuron®) was about 2.1 μM. The results are shown in Table 1.
Acknowledgments
This study was supported by the Ministry of Education, Youth and Sports of the Czech Republic (LO1305). The HPLC/HRMS system forms a part of the National Infrastructure CzeCOS ProCES CZ.02.1.01/0.0/0.0/16_013/0001609; M.O. was supported by the National Sustainability Program (NPU I; grant number LO1415).
References
- Lemke, T.L.; Williams, D.A. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins and Wolters Kluwer: Baltimore, MD, USA, 2013. [Google Scholar]
- Laursen, J.S.; Engel-Andreasen, J.; Fristrup, P.; Harris, P.; Olsen, C.A. Cis-trans amide bond rotamers in β-peptoids and peptoids: Evaluation of stereoelectronic effects in backbone and side chains. J. Am. Chem. Soc. 2013, 135, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Tang, K.C.; Raj, M. Amide bond activation of biological molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Pauk, K.; Imramovsky, A.; Vinsova, J.; Cizek, A.; Jampilek, J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed. Res. Int. 2015, 2015, 349534. [Google Scholar] [CrossRef] [PubMed]
- Good, N.E. Inhibitors of the Hill reaction. Plant Physiol. 1961, 36, 788–803. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibitory effects of substituted benzanilides on photosynthetic electron transport in spinach chloroplasts. Chem. Pap. 1999, 53, 328–331. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibition of photosynthetic electron transport in spinach chloroplasts by 3- and 4-halogeno substituted benzanilides and thiobenzanilides. J. Trace Microprobe Technol. 2000, 18, 251–256. [Google Scholar]
- Musiol, R.; Tabak, D.; Niedbala, H.; Podeszwa, B.; Jampilek, J.; Kralova, K.; Dohnal, J.; Finster, J.; Mencel, A.; Polanski, J. Investigating biological activity spectrum for novel quinoline analogues 2: Hydroxyquinolinecarboxamides with photosynthesis inhibiting activity. Bioorg. Med. Chem. 2008, 16, 4490–4499. [Google Scholar] [CrossRef]
- Kralova, K.; Perina, M.; Waisser, K.; Jampilek, J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015, 11, 156–164. [Google Scholar] [CrossRef]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. 1991, 3, 1621–1633. [Google Scholar] [CrossRef]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron-transport. Biochim. Biophys. Acta 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Advances in Pesticide Science; Greissbuehler, H., Ed.; Pergamon Press: Oxford, UK, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Izawa, S. Acceptors and donors for chloroplast electron transport. In Methods in Enzymology; Part C; Colowick, P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA; London, UK, 1980; Volume 69, pp. 413–434. [Google Scholar]
- Lambreva, M.D.; Russo, D.; Polticelli, F.; Scognamiglio, V.; Antonacci, A.; Zobnina, V.; Campi, G.; Rea, G. Structure/function/dynamics of photosystem II plastoquinone binding sites. Curr. Protein Pept. Sci. 2014, 15, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; de Andrade Barros, M.V.; Bressan, G.C.; Siqueira, R.P.; Dos Santos, F.S.; Bertazzini, M.; Kiralj, R.; Ferreira, M.M.C.; Forlani, G. Synthesis, theoretical studies, and effect on the photosynthetic electron transport of trifluoromethyl arylamides. Pest Manag. Sci. 2017, 73, 2360–2371. [Google Scholar] [CrossRef]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Kapustikova, I.; Clements, C.; Gray, A.I.; Jampilek, J. 3-Hydroxynaphthalene-2-carboxanilides and their antitrypanosomal activity. Monatsh. Chem. 2018, 149, 887–892. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-hydroxynaphthalene-2-carboxanilides affecting photosynthetic electron transport in photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Nevin, E.; Govender, R.; Pesko, M.; Kushkevych, I.; Oravec, M.; Kollar, P.; O´Mahony, J.; Kralova, K.; et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014, 19, 10386–10409. [Google Scholar] [CrossRef]
- Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O´Mahony, J.; et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767–9787. [Google Scholar] [CrossRef]
- Pesko, M.; Kos, J.; Kralova, K.; Jampilek, J. Inhibition of photosynthetic electron transport by 6-hydroxy- naphthalene-2-carboxanilides. Indian J. Chem. B 2015, 54B, 1511–1517. [Google Scholar]
- Jampilek, J.; Kralova, K.; Pesko, M.; Kos, J. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as photosystem II inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3862–3865. [Google Scholar] [CrossRef]
- Gonec, T.; Kralova, K.; Pesko, M.; Jampilek, J. Antimycobacterial N-alkoxyphenylhydroxynaphthalene- carboxamides affecting photosystem II. Bioorg. Med. Chem. Lett. 2017, 27, 1881–1885. [Google Scholar] [CrossRef]
- Gonec, T.; Stranik, J.; Pesko, M.; Kos, J.; Oravec, M.; Kralova, K.; Jampilek, J. Photosynthesis-inhibiting activity of 1-[(2-chlorophenyl)carbamoyl]- and 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl alkylcarbamates. Molecules 2017, 22, 1199. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Monreal-Ferriz, J.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef]
- Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Vejsova, M.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; et al. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012, 20, 7059–7068. [Google Scholar] [CrossRef]
- Imramovsky, A.; Pesko, M.; Jampilek, J.; Kralova, K. Synthesis and photosynthetic electron transport inhibition of 2-substituted 6-fluorobenzothiazoles. Monatsh. Chem. 2014, 145, 1817–1824. [Google Scholar] [CrossRef]
- Bak, A.; Pizova, H.; Kozik, V.; Vorcakova, K.; Kos, J.; Treml, J.; Odehnalova, K.; Oravec, M.; Imramovsky, A.; Bobal, P.; et al. SAR-mediated similarity assessment of property profile for new silicon-based AChE/BChE inhibitors. Int. J. Mol. Sci. 2019, 20, 5385. [Google Scholar] [CrossRef]
- Kralova, K.; Masarovicova, E.; Jampilek, J. Plant responses to stress induced by toxic metals and their nanoforms. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 479–522. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).