Adsorption of Rhodamine-B from Aqueous Solution by Activated Carbon from Almond Shell †
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Activated Carbon Preparation
3. Adsorption Experiments
4. Results and Discussion
5. Conclusions
References
- Butt, M.T.; Arif, F.; Shafique, T.; Imtiaz, N. Spectrophotometric estimation of colour in textile dyeing wastewater. J. Chem. Soc. Pak. 2005, 27, 627–630. [Google Scholar]
- Bao, L.; Meng, M.; Sun, K.; Li, W.; Zhao, D.; Li, H.; He, M. Selective adsorption and degradation of Rhodamine B with modified titanium dioxide photocatalyst. J. Appl. Polym. Sci. 2014, 131, 40896–40908. [Google Scholar] [CrossRef]
- Badmus, M.A.O.; Audu, T.O.K. Periwinkle shell: Based granular activated carbon for treatment of chemical oxygen demand (COD) in industrial wastewater. Can. J. Chem. Eng. 2009, 87, 69–77. [Google Scholar] [CrossRef]
- Klasson, K.T.; Wartelle, L.H.; Rodgers, J.E., III; Lima, I.M. Copper (II) adsorption by activated carbons from pecan shells: Effect of oxygen level during activation. Ind. Crops Prod. 2009, 30, 72–77. [Google Scholar] [CrossRef]
- Paraskeva, P.; Kalderis, D.; Diamadopoulos, E. Production of activated carbon from agricultural by-products. J.Chem. Technol. Biotechnol. 2008, 83, 581–592. [Google Scholar] [CrossRef]
- Montoya, V.H.; Bonilla-Petriciolet, A. (Eds.) Lignocellulosic Precursors Used in the Synthesis of Activated Carbon: Characterization Techniques and Applications in the Wastewater Treatment; InTech: Mexico, 2012. [Google Scholar]
- Mehrasbi, M.R.; Farahmandkia, Z.; Taghibeigloo, B.; Taromi, A. Adsorption of lead and cadmium from aqueous solution by using almond shells. Water Air Soil Pollut. 2009, 199, 343–351. [Google Scholar] [CrossRef]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
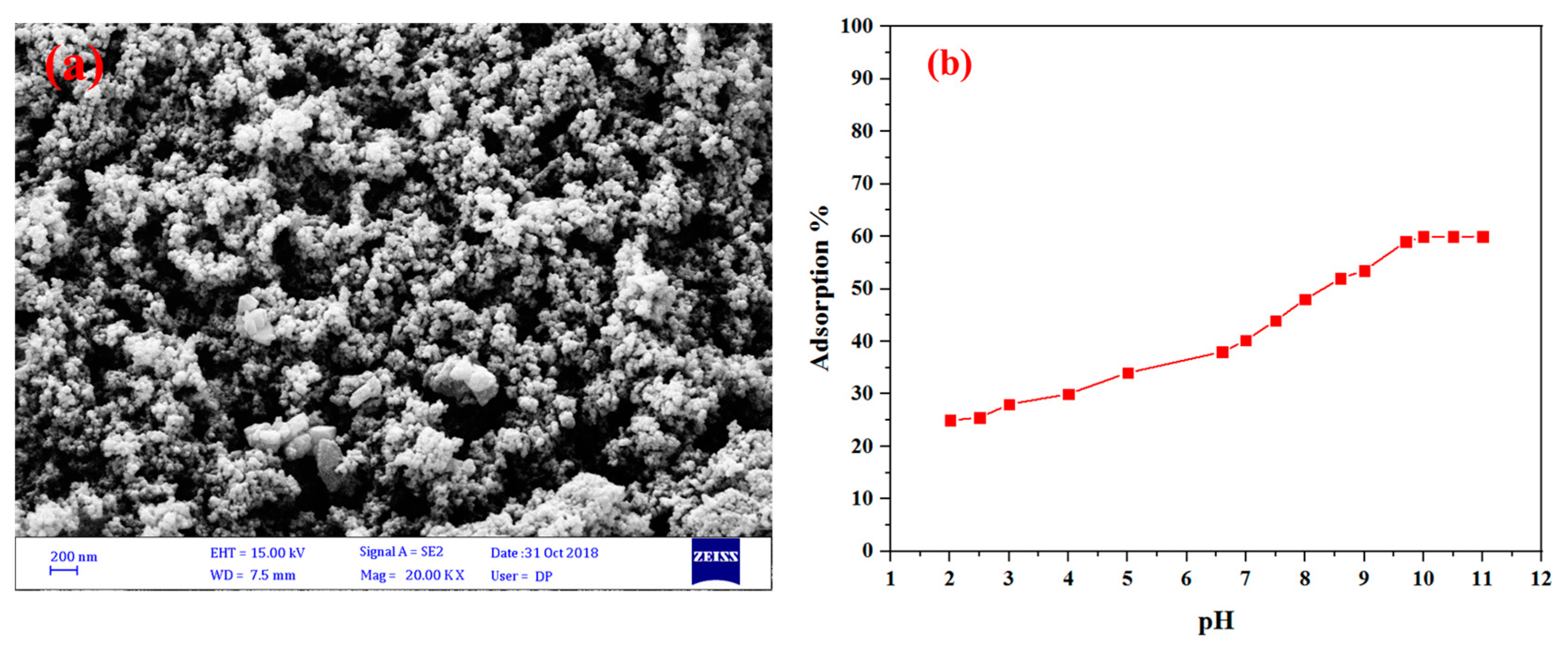
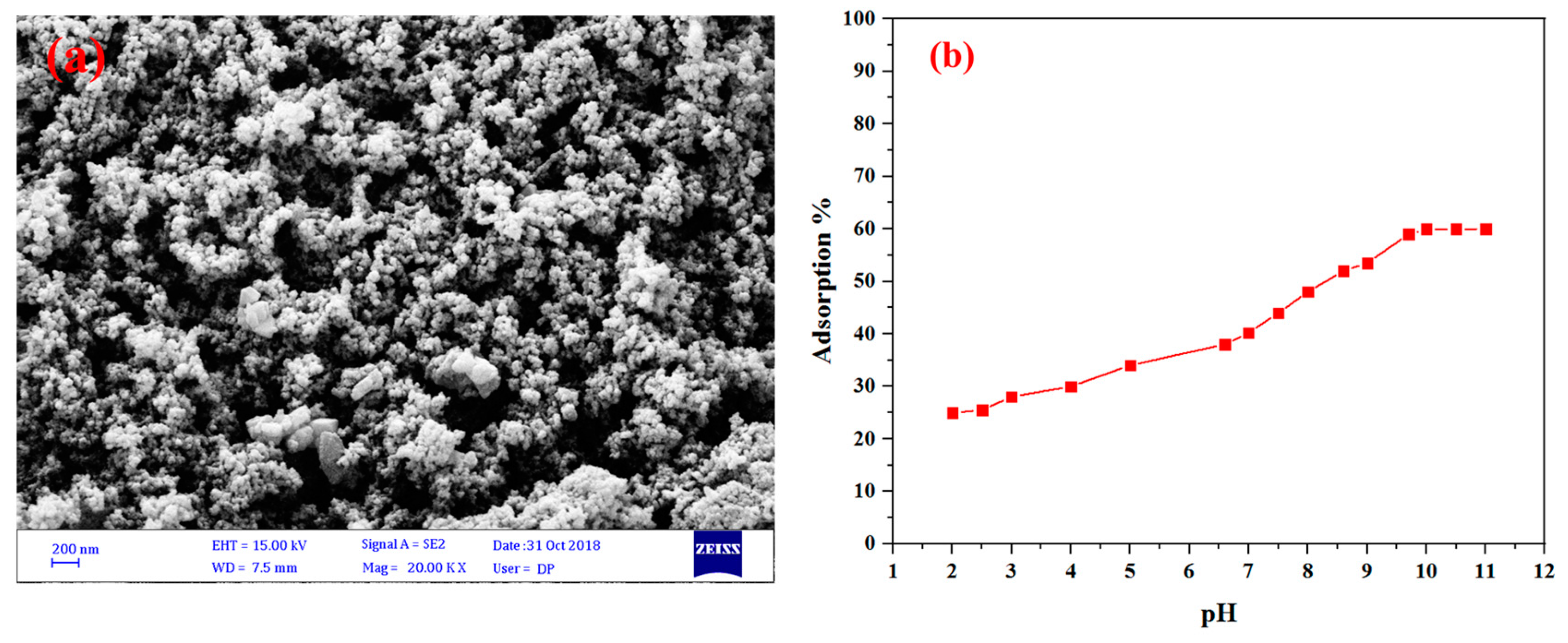
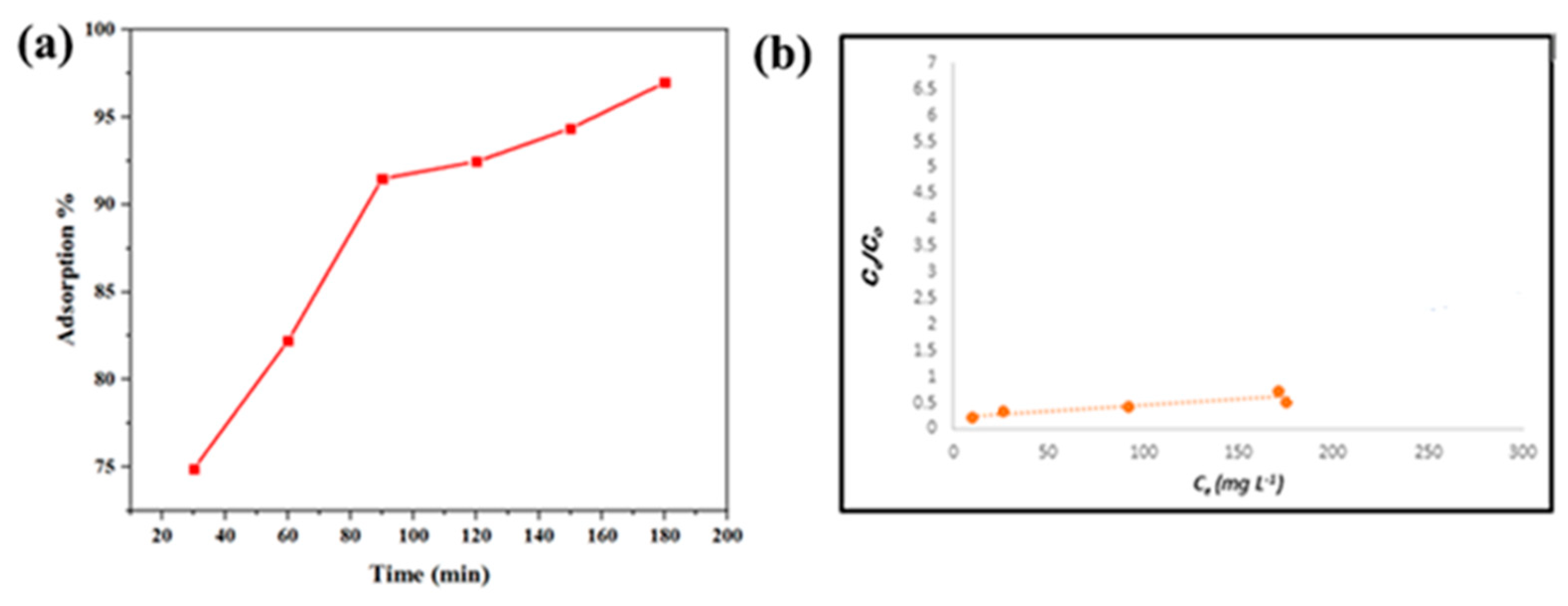
| Sample | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| Activated Carbon | 1252.23 | 1 | 4 |
| Sample | Qm (mg g−1) | Kl (L mg−1) | AL | R2 |
|---|---|---|---|---|
| Actived carbon | 255.39 | 2.488 | 0.017 | 0.930 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdolrahimi, N.; Tadjarodi, A. Adsorption of Rhodamine-B from Aqueous Solution by Activated Carbon from Almond Shell. Proceedings 2019, 41, 51. https://doi.org/10.3390/ecsoc-23-06619
Abdolrahimi N, Tadjarodi A. Adsorption of Rhodamine-B from Aqueous Solution by Activated Carbon from Almond Shell. Proceedings. 2019; 41(1):51. https://doi.org/10.3390/ecsoc-23-06619
Chicago/Turabian StyleAbdolrahimi, Nasim, and Azadeh Tadjarodi. 2019. "Adsorption of Rhodamine-B from Aqueous Solution by Activated Carbon from Almond Shell" Proceedings 41, no. 1: 51. https://doi.org/10.3390/ecsoc-23-06619
APA StyleAbdolrahimi, N., & Tadjarodi, A. (2019). Adsorption of Rhodamine-B from Aqueous Solution by Activated Carbon from Almond Shell. Proceedings, 41(1), 51. https://doi.org/10.3390/ecsoc-23-06619




