Halloysite Nanotubes Modified by Chitosan as an Efficient and Eco-Friendly Heterogeneous Nanocatalyst for the Synthesis of Heterocyclic Compounds †
Abstract
:1. Introduction
2. Experimental
2.1. General
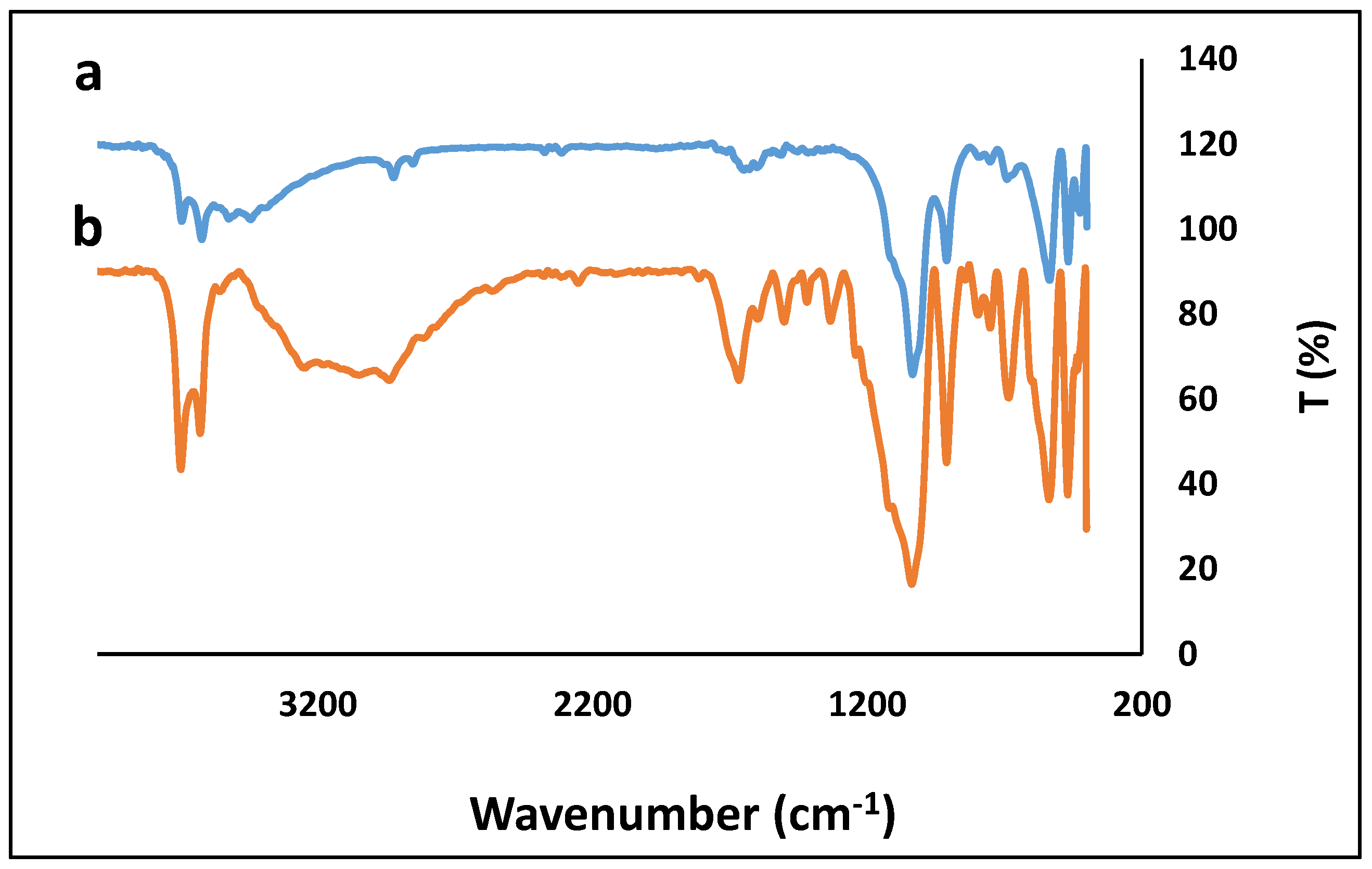
2.2. Synthesis of HNTs/Chit
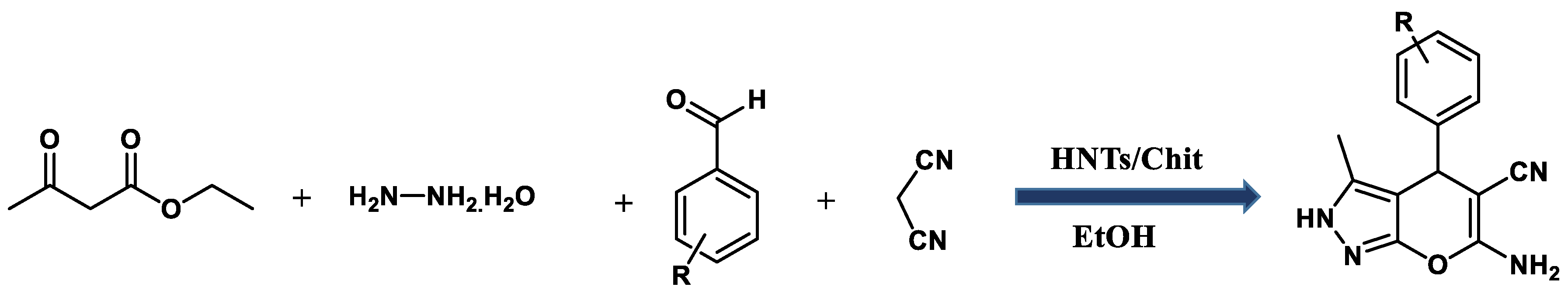
2.3. General Procedure for the Synthesis of Pyranopyrazole Derivatives 5a-e
3. Results and Discussion
3.1. Catalytic Application of HNTs/Chit in the Synthesis of Pyranopyrazole Derivatives
4. Conclusions
Acknowledgments
References
- Singh, B. Why Does Halloysite Roll?—A New Model. Clays Clay Miner. 1996, 44, 191–196. [Google Scholar] [CrossRef]
- Almasri, D.A.; Saleh, N.B.; Atieh, M.A.; McKay, G.; Ahzi, S. Adsorption of phosphate on iron oxide doped halloysite nanotubes. Sci. Rep. 2019, 9, 3232. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Tan, D.; Aannabi-Bergaya, F.; Yan, W.; Fan, M.; Liu, N.; He, H. Changes in Structure, Morphology, Porosity, and Surface Activity Of Mesoporous Halloysite Nanotubes Under Heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- Veerabadran, N.G.; Mongayt, D.; Torchilin, V.; Price, R.R.; Lvov, Y.M. Organized Shells on Clay Nanotubes for Controlled Release of Macromolecules. Macromol. Rapid Commun. 2009, 30, 99–103. [Google Scholar] [CrossRef]
- Hajizadeh, Z.; Maleki, A. Poly(ethylene imine)-modified magnetic halloysite nanotubes: A novel, efficient and recyclable catalyst for the synthesis of dihydropyrano[2,3-c]pyrazole derivatives. Mol. Catal. 2018, 460, 87–93. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan–halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 2078. [Google Scholar] [CrossRef]
- Soares, P.I.; Machado, D.; Laia, C.; Pereira, L.C.; Coutinho, J.T.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Thermal and magnetic properties of chitosan-iron oxide nanoparticles. Carbohydr. Polym. 2016, 149, 382–390. [Google Scholar] [CrossRef]
- Azzam, R.A.; Mohareb, R.M. Multicomponent Reactions of Acetoacetanilide Derivatives with Aromatic Aldehydes and Cyanomethylene Reagents to Produce 4H-Pyran and 1,4-Dihydropyridine Derivatives with Antitumor Activities. Chem. Pharm. Bull. 2015, 63, 1055–1064. [Google Scholar] [CrossRef]
- Van Leusen, A.; Siderius, H.; Hoogenboom, B.; Van Leusen, D. A new and simple synthesis of the pyrrole ring system from Michael acceptors and tosylmethylisocyanides. Tetrahedron Lett. 1972, 13, 5337–5340. [Google Scholar] [CrossRef]
- Liu, C.; Tan, Y.; Liu, C.; Chen, X.; Yu, L. Preparations, characterizations and applications of chitosan-based nanoparticles. J. Ocean Univ. China 2007, 6, 237–243. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Polysaccharides and Their Derivatives for Versatile Tissue Engineering Application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Zolfigol, M.A.; Tavasoli, M.; Moosavi-Zare, A.R.; Moosavi, P.; Kruger, H.G.; Shiri, M.; Khakyzadeh, V. Synthesis of pyranopyrazoles using isonicotinic acid as a dual and biological organocatalyst. RSC Adv. 2013, 3, 25681. [Google Scholar] [CrossRef]
- Maleki, A. Fe3O4/SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron 2012, 68, 7827–7833. [Google Scholar] [CrossRef]
- Maleki, A. One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Letters 2013, 54, 2055–2059. [Google Scholar] [CrossRef]
- Maleki, A. One-pot three-component synthesis of pyrido[2′,1′:2,3]imidazo[4,5-c]isoquinolines using Fe3O4@SiO2–OSO3H as an efficient heterogeneous nanocatalyst. RSC Adv. 2014, 4, 64169–64173. [Google Scholar] [CrossRef]
- Maleki, A. Synthesis of Imidazo[1,2- a ]pyridines Using Fe3O4@SiO2 as an Efficient Nanomagnetic Catalyst via a One-Pot Multicomponent Reaction. Helvetica Chim. Acta 2014, 97, 587–593. [Google Scholar] [CrossRef]
- Maleki, A. Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason. Sonochemistry 2018, 40, 460–464. [Google Scholar] [CrossRef]
- Maleki, A. An efficient magnetic heterogeneous nanocatalyst for the synthesis of pyrazinoporphyrazine macrocycles. Polycyclic Aromatic Compounds. 2018, 38, 402–409. [Google Scholar] [CrossRef]
- Maleki, A.; Ghassemi, M.; Firouzi-Haji, R. Green multicomponent synthesis of four different classes of six-membered N-containing and O-containing heterocycles catalyzed by an efficient chitosan-based magnetic bionanocomposite. Pure Appl. Chem. 2018, 90, 387–394. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Firouzi-Haji, R. Eco-friendly functionalization of magnetic halloysite nanotube with SO3H for synthesis of dihydropyrimidinones. Microporous Mesoporous Mater. 2018, 259, 46–53. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Abbasi, H. Surface modification of graphene oxide by citric acid and its application as a heterogeneous nanocatalyst in organic condensation reaction. Carbon Letters 2018, 27, 42–49. [Google Scholar]
- Maleki, A.; Ghalavand, R.; Firouzi-Haji, R. A novel and eco-friendly o-phenylendiamine stabilized on silica-coated magnetic nanocatalyst for the synthesis of indenoquinoline derivatives under ultrasonic-assisted solvent-free conditions. Iran. J. Catal. 2018, 8, 221–229. [Google Scholar]
- Maleki, A.; Akhlaghi, E.; Paydar, R. Design, synthesis, characterization and catalytic performance of a new cellulose-based magnetic nanocomposite in the one-pot three-component synthesis of α-aminonitriles. Applied Organometallic Chemistry. 2016, 30, 382–386. [Google Scholar] [CrossRef]
- Maleki, A.; Aghaei, M.; Ghamari, N. Facile synthesis of tetrahydrobenzoxanthenones via a one-pot three-component reaction using an eco-friendly and magnetized biopolymer chitosan-based heterogeneous nanocatalyst. Applied Organometallic Chemistry. 2016, 30, 939–942. [Google Scholar] [CrossRef]
- Maleki, A.; Rahimi, R.; Maleki, S. Efficient oxidation and epoxidation using a chromium (VI)-based magnetic nanocomposite. Environmental Chemistry Letters. 2016, 14, 195–199. [Google Scholar] [CrossRef]
- Maleki, A.; Movahed, H.; Ravaghi, P.; Kari, T. Facile in situ synthesis and characterization of a novel PANI/Fe3O4/Ag nanocomposite and investigation of catalytic applications. RSC Adv. 2016, 6, 98777–98787. [Google Scholar] [CrossRef]
- Maleki, A.; Aghaei, M.; Hafizi-Atabak, H.R. Ferdowsi, M. Ultrasonic treatment of CoFe2O4@ B2O3-SiO2 as a new hybrid magnetic composite nanostructure and catalytic application in the synthesis of dihydroquinazolinones. Ultrasonics Sonochemistry 2017, 37, 260–266. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A. Green and efficient synthesis of quinoxaline derivatives via ceric ammonium nitrate promoted and in situ aerobic oxidation of α-hydroxy ketones and α-keto oximes in aqueous media. Chemical and Pharmaceutical Bulletin 2008, 56, 79–81. [Google Scholar] [CrossRef]
- Shaabani, A.; Soleimani, E.; Maleki, A. One-pot three-component synthesis of 3-aminoimidazo [1, 2-a] pyridines and-pyrazines in the presence of silica sulfuric acid. Monatshefte für Chemie 2007, 138, 73–76. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A.; Behnam, M. Tandem oxidation process using ceric ammonium nitrate: Three-component synthesis of trisubstituted imidazoles under aerobic oxidation conditions. Synthetic Communications 2009, 39, 102–110. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z. Silicon 2019, 11, 2789–2798.

| Entry | R | Product | Yield a (%) | Mp (°C) |
|---|---|---|---|---|
| 1 | H | 5a | 95 | 244–246 |
| 2 | 3-NO2 | 5b | 93 | 234–235 |
| 3 | 4-NO2 | 5c | 91 | 247–249 |
| 4 | 4-Cl | 5d | 90 | 230–232 |
| 5 | 4-Me | 5e | 90 | 220–222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelodar, D.F.; Hajizadeh, Z.; Maleki, A. Halloysite Nanotubes Modified by Chitosan as an Efficient and Eco-Friendly Heterogeneous Nanocatalyst for the Synthesis of Heterocyclic Compounds. Proceedings 2019, 41, 59. https://doi.org/10.3390/ecsoc-23-06615
Jelodar DF, Hajizadeh Z, Maleki A. Halloysite Nanotubes Modified by Chitosan as an Efficient and Eco-Friendly Heterogeneous Nanocatalyst for the Synthesis of Heterocyclic Compounds. Proceedings. 2019; 41(1):59. https://doi.org/10.3390/ecsoc-23-06615
Chicago/Turabian StyleJelodar, Diana Fallah, Zoleikha Hajizadeh, and Ali Maleki. 2019. "Halloysite Nanotubes Modified by Chitosan as an Efficient and Eco-Friendly Heterogeneous Nanocatalyst for the Synthesis of Heterocyclic Compounds" Proceedings 41, no. 1: 59. https://doi.org/10.3390/ecsoc-23-06615
APA StyleJelodar, D. F., Hajizadeh, Z., & Maleki, A. (2019). Halloysite Nanotubes Modified by Chitosan as an Efficient and Eco-Friendly Heterogeneous Nanocatalyst for the Synthesis of Heterocyclic Compounds. Proceedings, 41(1), 59. https://doi.org/10.3390/ecsoc-23-06615






