Facile Fabrication of DNA Biosensors Based on Oxidized Carbon Black and Graphite Oxide †
Abstract
:1. Introduction
2. Experimental Process
2.1. The Materials and Methods Section
2.2. Synthesis of Graphite Oxide and Carbon Black Oxide
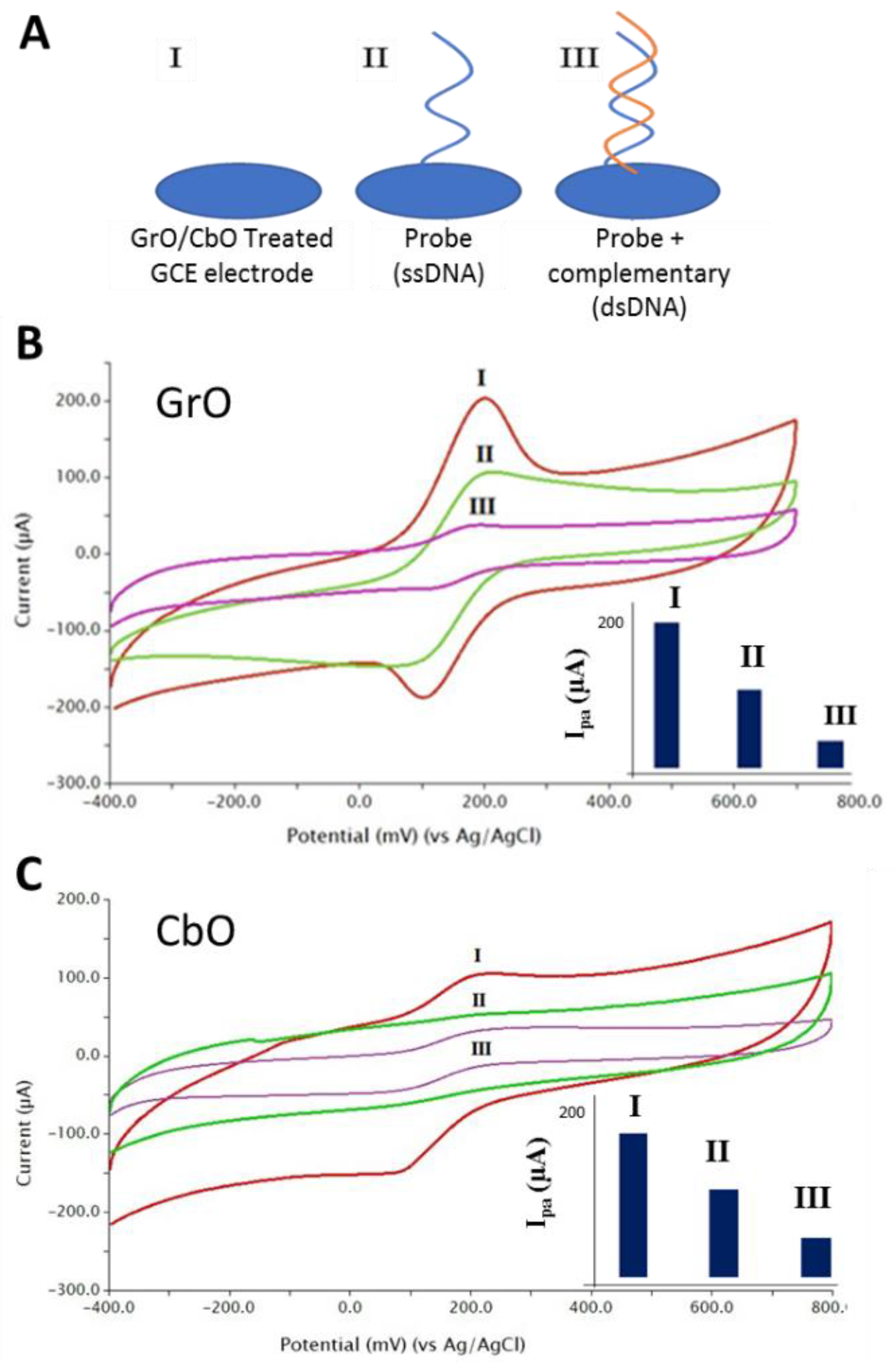
2.3. Covalently Immobilization of DNA
2.4. Electrochemical Detection
3. Result and Discussion
4. Conclusions
References
- Prajapati, D.G.; Kandasubramanian, B. Progress in the Development of Intrinsically Conducting Polymer Composites as Biosensors. Macromol. Chem. Phys. 2019, 220. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Eren, T.; Atar, N. A novel and sensitive electrochemical DNA biosensor based on Fe@ Au nanoparticles decorated graphene oxide. Electrochim. Acta 2014, 125, 38–47. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Aghaei, F.; Seifati, S.M.; Nasirizadeh, N. Development of a DNA biosensor for the detection of phenylketonuria based on a screen-printed gold electrode and hematoxylin. Anal. Methods 2017, 9, 966–973. [Google Scholar] [CrossRef]
- Beebe, T.P.; Rabke-Clemmer, C.E. Thiol Labeling of DNA for Attachment to Gold Surfaces. U.S. Patent 5472881A, 5 December 1995. [Google Scholar]
- Silva, M.M.; Cavalcanti, I.T.; Barroso, M.F.; Sales, M.G.F.; Dutra, R.F. Gold electrode modified by self-assembled monolayers of thiols to determine DNA sequences hybridization. J. Chem. Sci. 2010, 122, 911–917. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z. Carbon nanomaterial-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical biosensors based on nanostructured carbon black: A review. J. Nanomater. 2017. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.-I. Graphene oxide nanosheet with high proton conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-D.; Huang, J.; Liang, R.-P. Nanocomposite film based on graphene oxide for high performance flexible glucose biosensor. Sens. Actuators B Chem. 2011, 160, 287–294. [Google Scholar] [CrossRef]
- Rahimi, R.; Moshari, M.; Rabbani, M.; Azad, A. Photooxidation of benzyl alcohols and photodegradation of cationic dyes by Fe3O4 @ sulfur/reduced graphene oxide as catalyst. RSC Adv. 2016, 6, 41156–41164. [Google Scholar] [CrossRef]
- Wang, J.; Kirgöz, Ü.A.; Mo, J.-W.; Lu, J.; Kawde, A.N.; Muck, A. Glassy carbon paste electrodes. Electrochem. Commun. 2001, 3, 203–208. [Google Scholar] [CrossRef]
- Okahata, Y.; Kobayashi, T.; Tanaka, K.; Shimomura, M. Anisotropic electric conductivity in an aligned DNA cast film. J. Am. Chem. Soc. 1998, 120, 6165–6166. [Google Scholar] [CrossRef]
- Semenza, G.L.; Prabhakar, N.R. The role of hypoxia-inducible factors in oxygen sensing by the carotid body. In Arterial Chemoreception; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–5. [Google Scholar]
- LaBuda, C.J.; Fuchs, P.N. Aspirin attenuates the anxiolytic actions of ethanol. Alcohol 2000, 21, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Moshari, M.; Rabbani, M.; Rahimi, R. Synthesis of TCPP–Fe3O4 @ S/RGO and its application for purification of water. Res. Chem. Intermed. 2016, 42, 5441–5455. [Google Scholar] [CrossRef]
- Rahimi, R.; Moshari, M.; Rabbani, M. Photocatalytic Degredration of Methylene Blue and Crystal Violet by Sulfur/Reduced Graphene Oxide Composite. In Proceedings of the 18th International Electronic Conference on Synthetic Organic Chemistry, 1–30 November 2014. [Google Scholar]
- Gudarzi, M.M.; Sharif, F. Enhancement of dispersion and bonding of graphene-polymer through wet transfer of functionalized graphene oxide. Express Polym. Lett. 2012, 6, 1017–1031. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Hu, A.; Lu, Y.; Shi, F.; Lei, B.; Sun, Z. Electrochemical DNA biosensor based on partially reduced graphene oxide modified carbon ionic liquid electrode for the detection of transgenic soybean A2704-12 gene sequence. Electroanalysis 2013, 25, 1417–1424. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Ju, X.; Li, G.; Gao, H.; Sun, Z. Electrochemical deoxyribonucleic acid biosensor based on carboxyl functionalized graphene oxide and poly-l-lysine modified electrode for the detection of tlh gene sequence related to vibrio parahaemolyticus. Anal. Chim. Acta 2012, 752, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.; Ban, L.; McDonald, G. Carbon black morphology: I. Particle microstructure. II. Automated EM analysis of aggregate size and shape. Rubber Chem. Technol. 1969, 42, 1209–1234. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moshari, M.; Koirala, D.; Allen, P.B. Facile Fabrication of DNA Biosensors Based on Oxidized Carbon Black and Graphite Oxide. Proceedings 2019, 41, 70. https://doi.org/10.3390/ecsoc-23-06612
Moshari M, Koirala D, Allen PB. Facile Fabrication of DNA Biosensors Based on Oxidized Carbon Black and Graphite Oxide. Proceedings. 2019; 41(1):70. https://doi.org/10.3390/ecsoc-23-06612
Chicago/Turabian StyleMoshari, Mahsa, Dipak Koirala, and Peter B. Allen. 2019. "Facile Fabrication of DNA Biosensors Based on Oxidized Carbon Black and Graphite Oxide" Proceedings 41, no. 1: 70. https://doi.org/10.3390/ecsoc-23-06612



