Photochromic Azoderivatives for Acetylcholinesterase Inhibition †
Abstract
:1. Introduction
2. Experimental Procedure
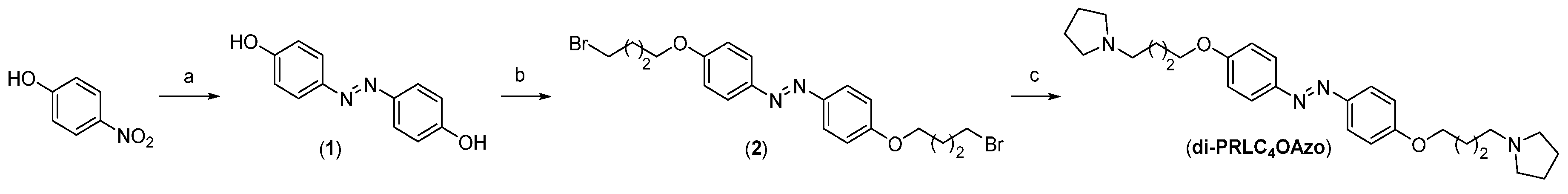
2.1. Materials and Synthesis
2.2. Methods
2.3. Results and Discussion
3. Conclusions
Acknowledgments
References
- McHardy, S.; Wang, H.; McCowen, S.; Valdez, M. Recent advances in acetylcholinesterase Inhibitors and Reactivators: An update on the patent literature (2012–2015). Expert Opin. Ther. Pat. 2017, 27, 455–476. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wehle, S.; Kuzmanovic, N.; Merget, B.; Holzgrabe, U.; König, B.; Sotriffer, C.A.; Decker, M. Acetylcholinesterase Inhibitors with Photoswitchable Inhibition of β-Amyloid Aggregation. ACS Chem Neurosci. 2014, 5, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, B.; Kuzmanovic, N.; Löffler, P.; Merkl, R.; König, B.; Sterner, R. Exploiting protein symmetry to design light-controllable enzyme inhibitors. Angew. Chem. Int. Ed. 2014, 53, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Broichhagen, J.; Frank, J.A.; Trauner, D. A roadmap to success in photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Broichhagen, J.; Jurastow, I.; Iwan, K.; Kummer, W.; Trauner, D. Optical Control of Acetylcholinesterase with a Tacrine Switch. Angew. Chem. Int. Ed. 2014, 53, 7657–7660. [Google Scholar] [CrossRef] [PubMed]
- Agnetta, C.; Decker, M. Photoresponsive Hybrid Compounds. In Design of Hybrid Molecules for Drug Development, 1st ed.; Decker, M., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 11, pp. 279–315. [Google Scholar]
- Biscussi, B.; Menéndez, C.; Appignanesi, G.A.; Gerbino, D.C.; Murray, A.P. XXI SINAQO; Organizado por la Sociedad Argentina de Investigación en Química Orgánica (SAIQO): Potrero de los Funes, San Luis, Argentina, 2017; p. 239. [Google Scholar]
- Sequeira, M.A.; Herrera, G.; Quirolo, Z.; Dodero, V. Easy Directed Assembly of only Nonionic Azoamphiphile Builds up Functional Azovesicles. RSC Adv. 2016, 6, 108132–108135. [Google Scholar] [CrossRef]
- Okuno, H.; Wei, W.H.; Tomohiro, T.; Kodaka, M. Selective Synthesis and Kinetic Measurement of 1:1 and 2:2 Cyclic Compounds Containing 1, 4, 7, 10-Tetraazacyclododecane and Azobenzene Units. J. Org. Chem. 2000, 65, 8979–8987. [Google Scholar]
- Willstatter, R.; Benz, M. Zur Kenntniss der ‘Azophenole. Chem. Ber. 1906, 339, 3492. [Google Scholar] [CrossRef]
- Jaeger, C. Ueber das Azophenol. Berichte Der Deutschen Chemischen Gesellschaft 1875, 8, 1499. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Alza, N.P.; Richmond, V.; Baier, C.J.; Freire, E.; Baggio, R.; Murray, A.P. Synthesis and cholinesterase inhibition of cativic acid derivatives. Org. Med. Chem. 2014, 22, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- Benedini, L.A.; Sequeira, M.A.; Fanani, M.L.; Maggio, B.; Dodero, V.I. Development of a Nonionic Azobenzene Amphiphile for Remote Photocontrol of a Model Biomembrane. J. Phys. Chem. B 2016, 120, 4053–4063. [Google Scholar] [CrossRef] [PubMed]
- Biscussi, B.; Murray, A.P. Síntesis de análogos de aza-resveratrol con actividad anticolinesterasa. In Proceedings of the XXVI JJI Jornada de Jóvenes Investigadores AUGM, Universidad Nacional de Cuyo, Mendoza, Argentina, 17–19 October 2018; Available online: http://bdigital.uncu.edu.ar/13200 (accessed on 8 November 19).


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscussi, B.; Sequeira, M.A.; Murray, A.P. Photochromic Azoderivatives for Acetylcholinesterase Inhibition. Proceedings 2019, 41, 80. https://doi.org/10.3390/ecsoc-23-06518
Biscussi B, Sequeira MA, Murray AP. Photochromic Azoderivatives for Acetylcholinesterase Inhibition. Proceedings. 2019; 41(1):80. https://doi.org/10.3390/ecsoc-23-06518
Chicago/Turabian StyleBiscussi, Brunella, Maria Alejandra Sequeira, and Ana Paula Murray. 2019. "Photochromic Azoderivatives for Acetylcholinesterase Inhibition" Proceedings 41, no. 1: 80. https://doi.org/10.3390/ecsoc-23-06518
APA StyleBiscussi, B., Sequeira, M. A., & Murray, A. P. (2019). Photochromic Azoderivatives for Acetylcholinesterase Inhibition. Proceedings, 41(1), 80. https://doi.org/10.3390/ecsoc-23-06518






