Abstract
Silica gel was used as an adsorbent for dyes in aqueous solutions. Afterwards, the silica gel with the adsorbed dye was heated to 600 °C, at which the dye combusted, leaving behind clean silica gel. This silica gel can be reused in the adsorption process. The operation leaves behind little waste products. It is an optimal procedure for educational and other research laboratories which are working with biological stains, food colorants and some non-commercial dyes.
1. Introduction
Remnant dye solutions that are generated by small laboratories are regarded as chemical waste that cannot be disposed of down the sink and into the sanitary sewer system. Instead, they must be sent to specialized waste stream companies. This way of disposal can be rather expensive to small laboratories, given that they have limited budgets. However, there is also silica gel waste generated that adds to the expenses of chemical waste disposal. On the other hand, silica gel can act as an adsorbent for a good number of the dyes used in these laboratories. So, if the silica gel is recycled and used for adsorption of the remnant dye solutions, we can cut down on the expenses of treating chemical waste.
Adsorption occurs when the adsorbate (gas or liquid) is captured in the pores of the adsorbent as a result of Van der Waals and/or electrostatic forces. Thanks to their high surface area and large pore volumes [1], silica gels are considered to be effective adsorbents [2], especially for the removal of dyes [3]. In this research, mostly cationic dyes have been studied, with Neutral Red being a neutral dye. Adsorption patterns depend on the dyes and the type of silica used. For cationic dyes, an electrostatic interaction occurs between the positive charges of the dye, and the oxygen atoms and hydroxyl groups of the silica [1,3]. Then, the adsorption can be detected by UV-spectroscopy over time as the adsorption progresses, and the information retrieved can be used to construct adsorption isotherms. Adsorption isotherms can then be studied to understand the adsorption process and to measure the decrease in the concentration of the dye as it adsorbs to the surface of the silica gel.
In this paper, we propose a process of dye adsorption using silica gel as an adsorbent, where after the adsorption process we filter the solution that contains the silica gel with the adsorbed dye, and then heat the silica gel with the adsorbed dye in an oven to 550–600 °C. The adsorbed dye will combust at that temperature to produce clean silica gel that has been found to be re-usable with the same efficiency as the non-recycled silica gel.
Moreover, we studied the effect of temperature on the adsorption process and on the maximum absorption capacity of the silica gel for the respective dye. We also studied potential changes in the silica gel, including its ability to adsorb the dye from an aqueous solution, each time the silica gel was recycled. Lastly, we embedded our research in context of a life cycle assessment of the silica gel and an environmental impact assessment to evaluate how much is really saved in terms of environmental and energy costs. Here, it has to be noted that silica gel of Merck grade 9385 is used in quantities of about 30 kg per year in our research laboratory as a stationary phase in the column chromatographic separation of mixtures stemming from organic reactions, where the silica gel is also recycled by thermolysis at 600 °C. The environmental impact assessment of the recycling of silica gel used for chromatographic purposes in a small research and teaching laboratory has already been communicated [4]. Here, it was found that the same recycled silica gel can be used for the treatment of remnant dye solutions as shown in this paper, which adds to the previous environmental impact assessment. Its prior use in the chromatographic separation of organic reaction mixtures does not affect its ability to adsorb dyes after the silica gel has been recycled thermally.
While we have looked at many different dyes in this process, in this paper we will focus on the adsorption of Neutral Red from aqueous solutions using pristine and recycled silica gel, originally of Merck grade 9385 (fine, pore size 60 Å, 230–400 mesh, bp. 2230 °C, m.p. > 1600 °C) and of grade Aldrich 60742 (coarse, pore size 60 Å, 35–60 mesh, bp. 2230 °C, m.p. > 1600 °C).
2. Materials and Methods
Silica gel of Merck grade 9385 (fine, pore size 60 Å, 230–400 mesh, bp. 2230 °C, m.p. > 1600 °C) and silica gel Aldrich 60742 (coarse, pore size 60 Å, 35–60 mesh, bp. 2230 °C, m.p. > 1600 °C) were used in the experiments. For the recycling of the silica gel, a Carbolite electrical oven ELF 11-6 was used. The oven was heated to 550–600 °C. After 2 h, the oven was shut off, and the inner temperature was allowed to return to 30 °C. Afterwards, the recycled silica gel was sieved through a Retsch sieve with mesh of 125 μm aperture. Although smaller amounts of silica gel are often recycled, the environmental impact assessment was carried out on 120 g silica gel batches, which are generally also used in our laboratory for the recycling of solvent-loaded silica gel (after column chromatography). The dyes used such as Neutral Red (Sigma), Toluidine Blue O (Aldrich), Fast Green FCF (Fluka) and Nitrazene (BDH) are all commercial products. Typically, a stock solution of dye (e.g., of Neutral Red and Toluidine Blue O) was prepared by dissolving 50 mg of dye in 500 mL of water. Then, we treated 100 mL of the dye solution (100 ppm) with 0.5 g of silica gel (Merck grade 9385). The temperature of the solution was adjusted 25 °C (rt). The solution was stirred for 30 min, and aliquots of the liquid were taken and centrifuged in a Beckman TJ-6 centrifuge at 1500 rpm for 5 min. The residual dye concentration was determined by UV spectroscopy using a Cary 50 instrument. This process was repeated at least four time to be able to establish a proper kinetic curve. Then, the silica (which had adsorbed the dye at this stage) was filtered off. The remaining aqueous solution was discarded into a sink as normal. The silica gel was transferred into a porcelain crucible. The crucible was heated in an oven to 600 °C for 2 h (at this point, the adsorbed dye combusts). The cooled silica gel was re-used in the next purification cycle.
For waste management of residual dye solutions in the laboratory, typically 0.5 g silica gel are added to 250 mL of dye solution (@50–100 ppm of the dye). The mixture is stirred on a magnetic stirrer for 10 min, and the silica gel is filtered over a glass fritte, after which the filtrate is discarded in a sink. The filtered silica gel is collected and heated in a Carbolite electrical oven ELF 11-6 and reused.
3. Results and Discussion
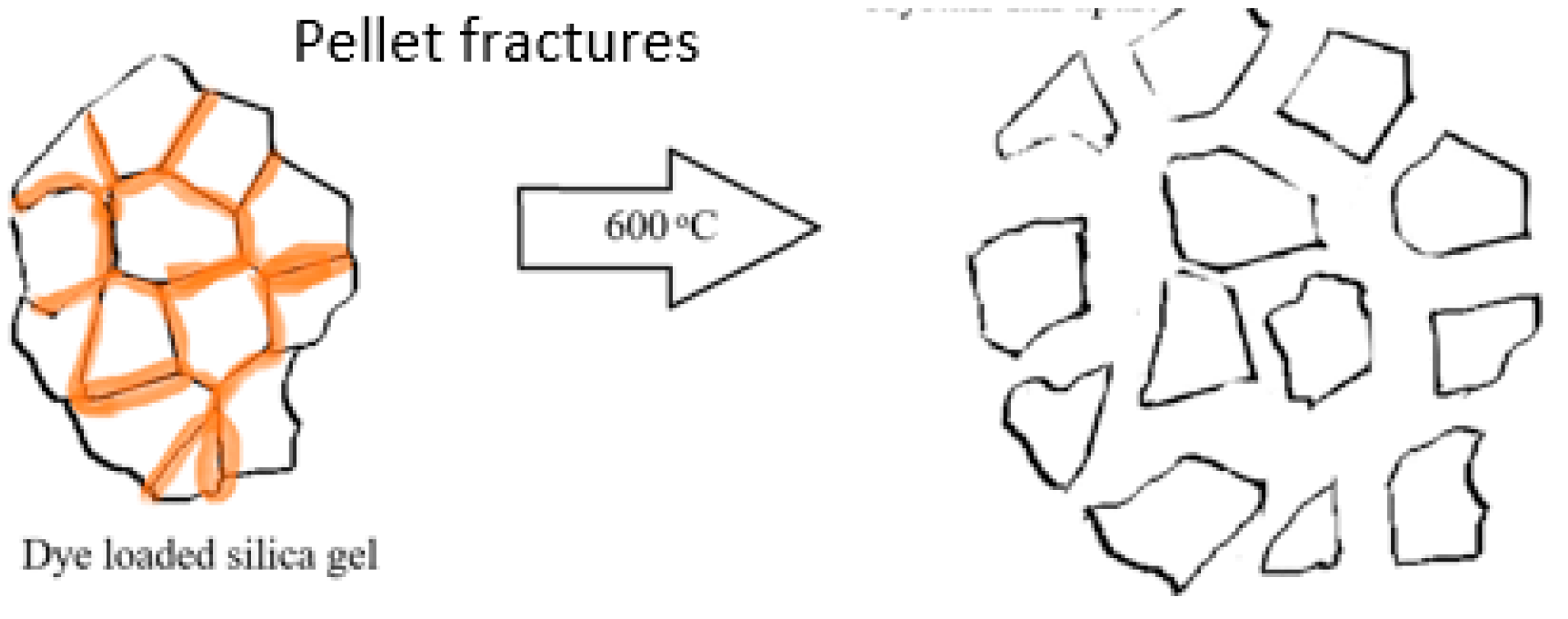
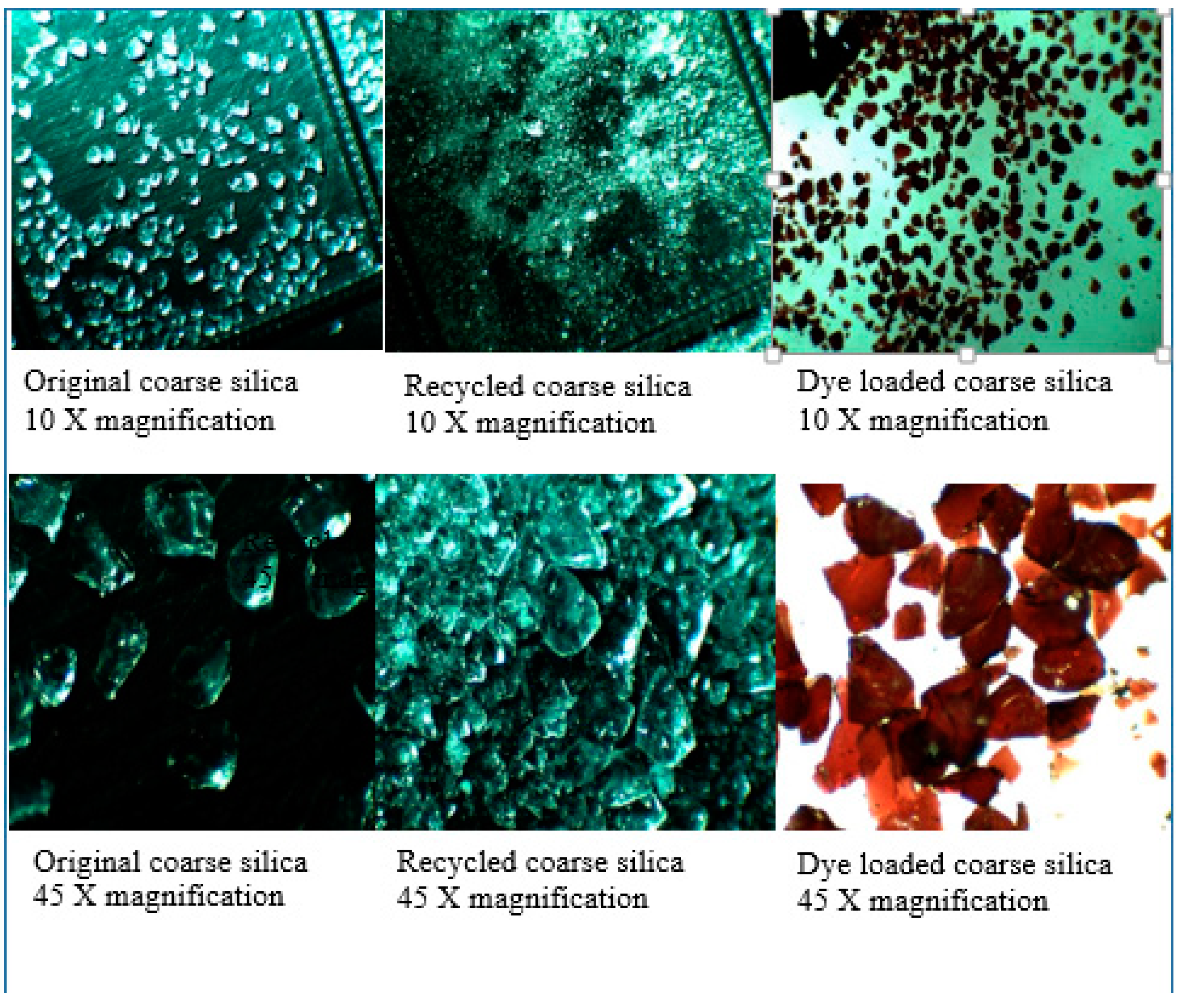
Silica gel—the properties of the silica material used, both pristine and recycled, have been discussed previously [4]. In brief, SEM studies of the original silica gel Merck grade 9385 and of the recycled silica gel Merck grade 9385 (e.g., 5× recycled) show no noticeable difference in size, shape or morphology. This is supported by Brunauer–Emmett–Teller (BET) surface measurements with a BET surface area of 394.5 m2/g for the original silica gel, 387.8 m2/g for 1× recycled silica gel and 422.3 m2/g for 2× recycled silica gel, initially showing a slight decrease in BET surface area, but then a slight increase after the second recycling. The average (adsorption) pore diameter (4 V/A) decreases, however, from 78.5 Å of the original silica gel over 72.7 Å (for 1× recycled silica gel) to 65.9 Å (for 2× recycled silica gel). For the coarse silica gel Aldrich 60742, it was found that the silica crystallites (Figure 1) seem to have been pelletized as the recycling of the silica gel led to the fracturing of these pellets into small pieces (see Figure 2, showing photos taken under the optical microscope). After the pristine silica gel had been recycled once, no further changes in size or shape of the silica particles occurred upon further recycling. It was noted that the dye that penetrated into the pellet along the surfaces of the crystallites may be responsible for fracturing the pellets where released gases during the combustion process exerted internal pressure on the pellet (Figure 1).
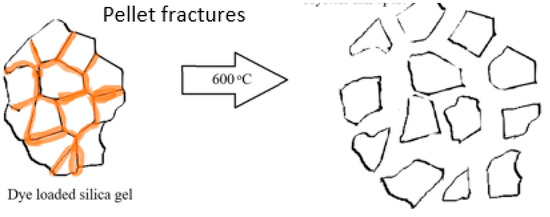
Figure 1.
Schematic of fracturing of pelleted silica gel Aldrich 60742 during the combustion of a dye at 600 °C in an oven.
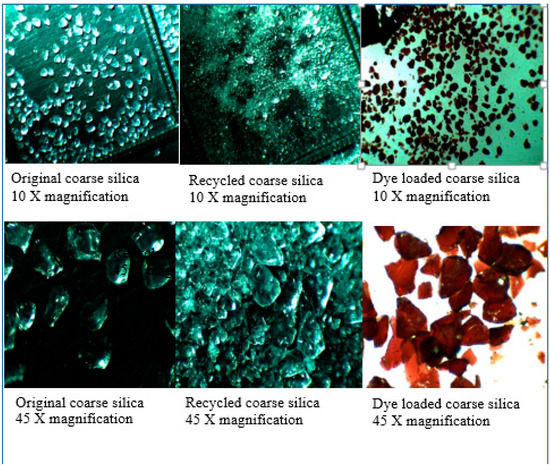
Figure 2.
Photos of silica gel Aldrich 60742 under the optical microscope—the original is on the left, the recycled silica gel is at the center; and the recycled, dye-loaded silica gel is on the right.
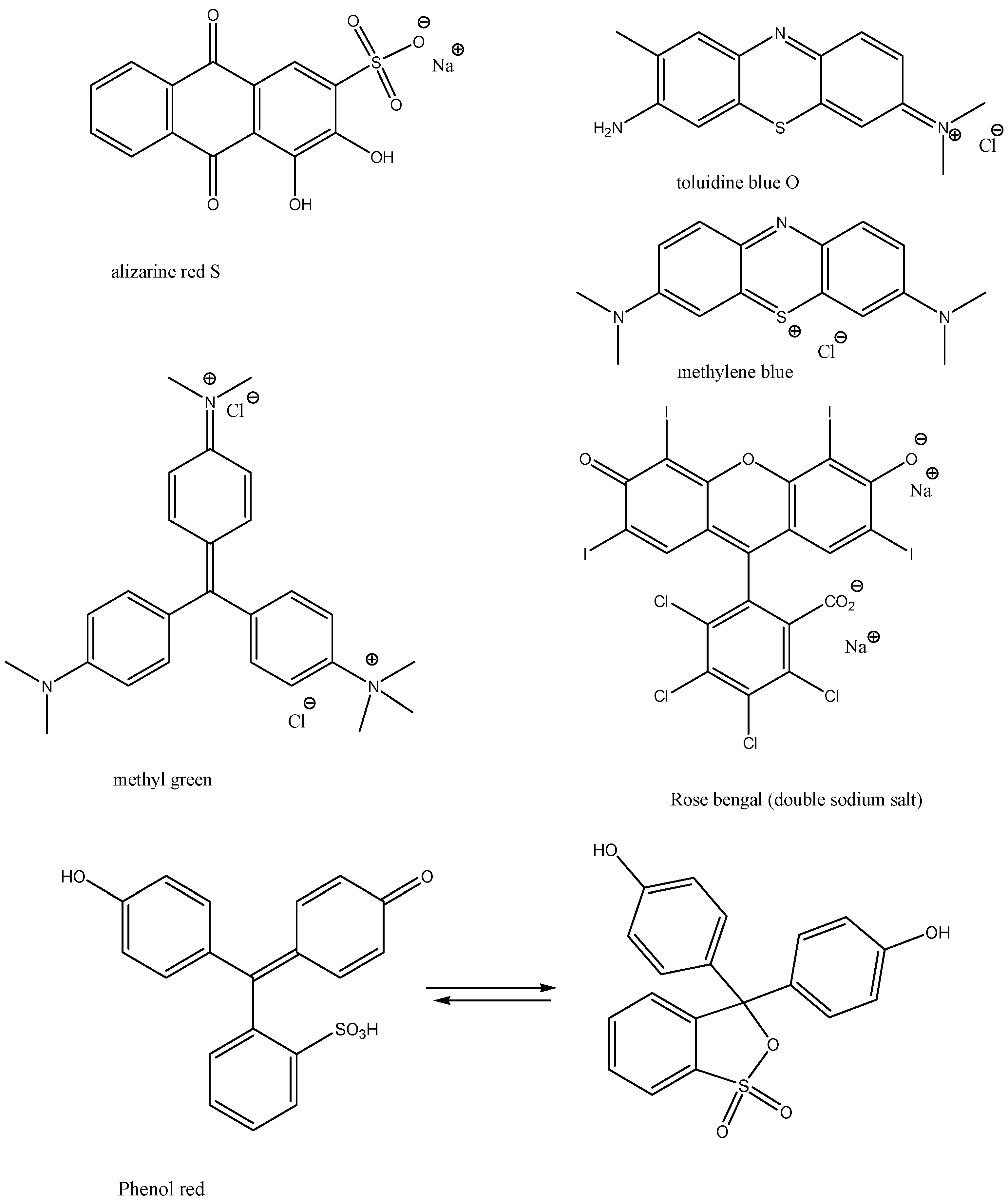
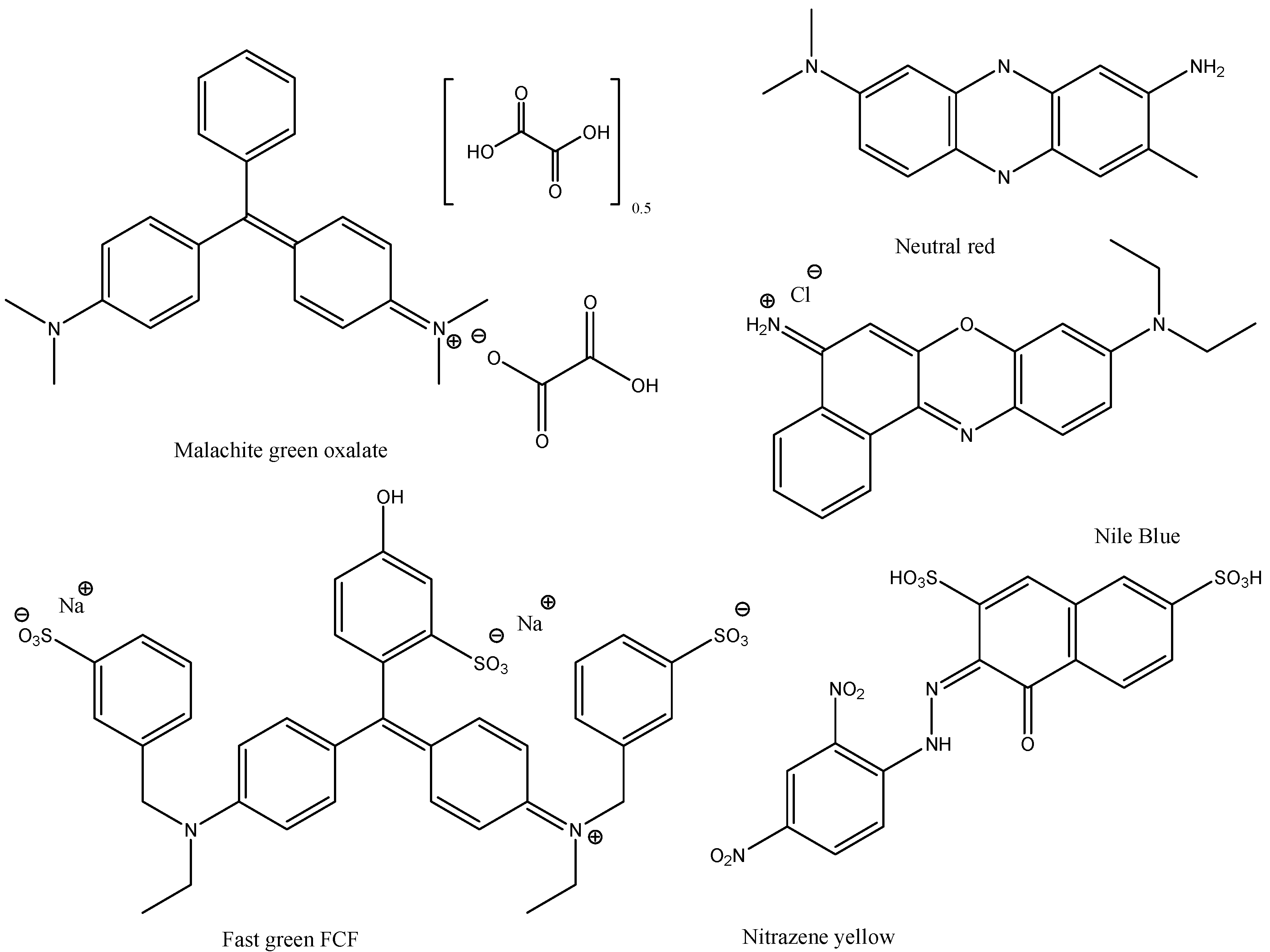
Dyes and stains—we chose the following stains, food colorants and dyes: Methylene Blue, Malachite Green Oxalate, Neutral Red, Toluidine Blue O, Methyl Green, Rose Bengal, Phenol Red, Alizarine Red S, Fast Green FCF, Nitrazene Yellow, Nile Blue, and Rhodamine B (Figure 3 and Figure 4). These dyes and stains were selected as they are some of the most commonly used in educational laboratories.
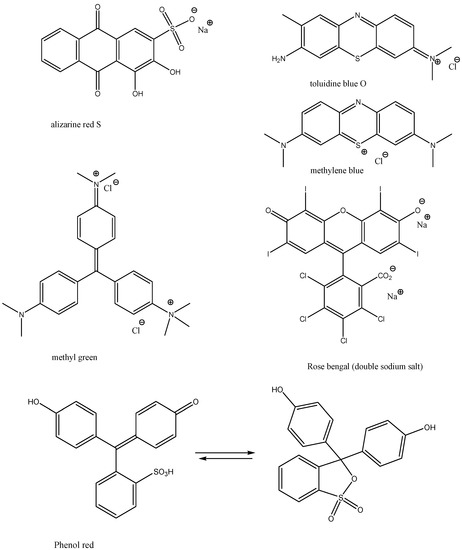
Figure 3.
Structures of dyes and stains investigated in this study.
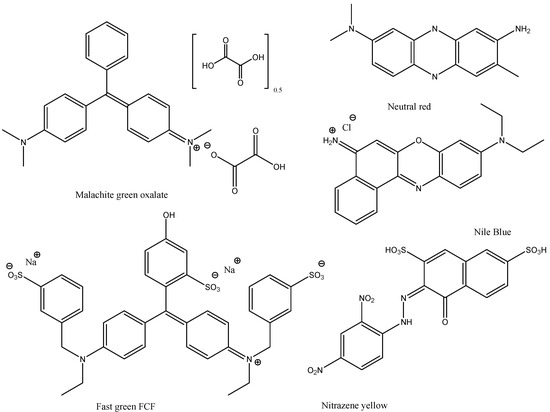
Figure 4.
Structures of dyes and stains investigated in this study (continued).
Toluidine Blue O, Malachite Green, Methylene Blue and Neutral Red all exhibit an aniline function, either free (Toluidine Blue O) or methylated. All these compounds exhibit rapid adsorption on the relatively acidic silica gel. In the case of Malachite Green and Neutral Red, the silica gel was loaded and recycled five times without any noticeable deterioration in the silica gel as an adsorbent.
Alizarine S was found not to adsorb significantly into silica gel but into basic alumina instead (Brockmann activity 1). Nitrazene Yellow (phenaphthazine) is known as an indicator dye. In our experiments, it has been found not to show significant adsorption into silica gel nor into basic alumina (Brockmann activity 1). When silica gel (1 g) was added to a 100 ppm aqueous solution of Nitrazene Yellow, the color of the solution changed from dark blue to yellow, showing the acidity of the silica gel. The use of 5 g silica gel or of basic alumina had little noticeable effect on the Nitrazene concentration in the aqueous solution, so both silica gel and basic alumina are not seen to be adequate adsorption media for Nitrazene Yellow.
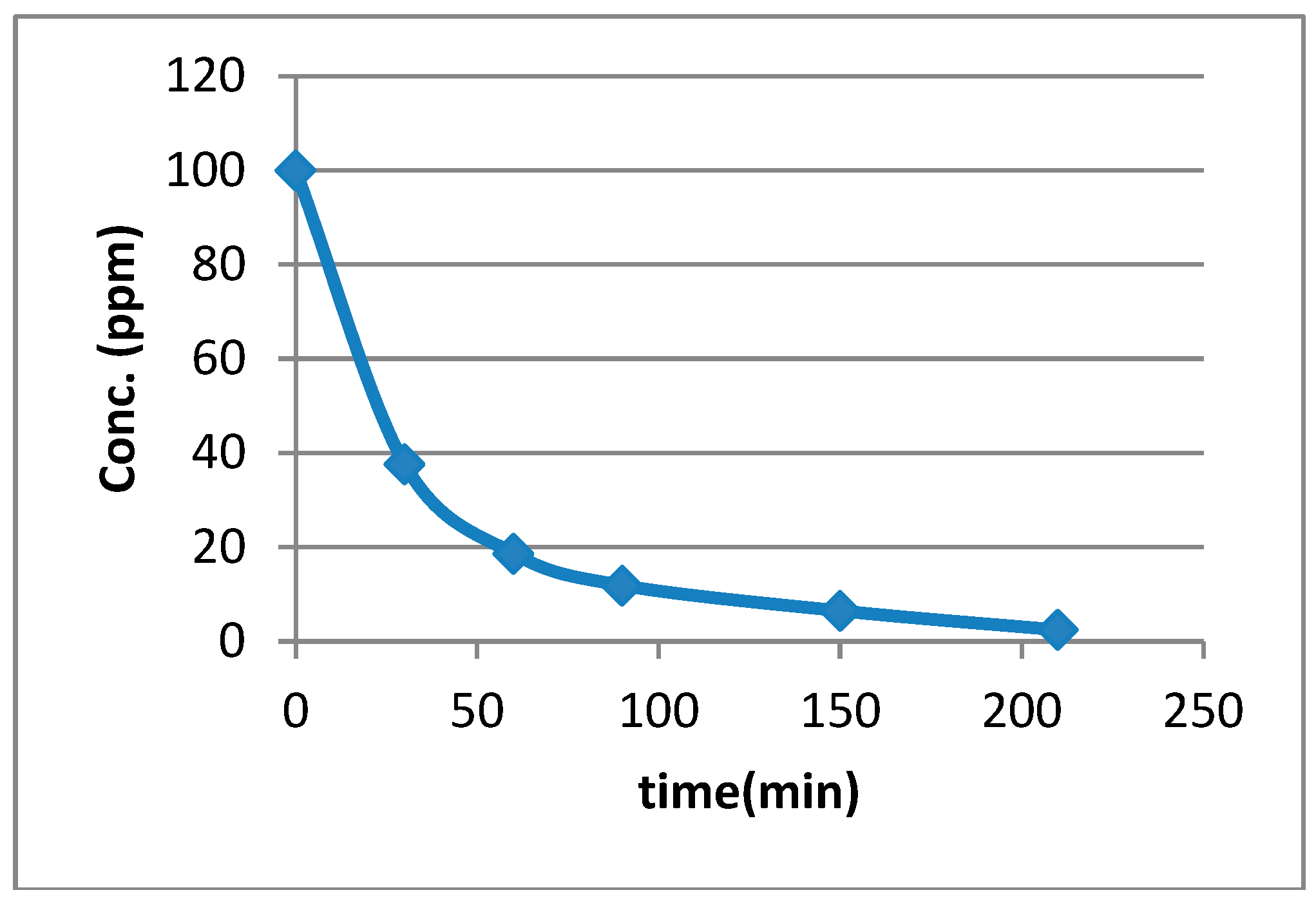
An example of an adsorption study with Neutral Red—Adsorption of most dyes studied on silica gel of Merck grade 9385 (fine, pore size 60 Å, 230–400 mesh) was so rapid that no kinetic studies were performed for most dyes. This allows, however, for the use of silica gel of Merck grade 9385 in the simple and speedy treatment of remnant dye solutions in educational and small research laboratories. The adsorption of dyes on silica gel Aldrich 60742 proceeded more slowly. Here, it was possible to investigate adsorption kinetics. Figure 5 shows the diminishing concentration of the dye Neutral Red in aqueous solution, indicating the time take for the dye to be adsorbed into silica gel Aldrich 60742. The concentration of the dye was seen to decrease from 100 ppm to 37 ppm after 30 min, and to 2.5 ppm after 210 min (3 h and 30 min). We recycled the silica and reused it for another adsorption process without seeing a marked difference in the adsorption behavior of the dye. Using silica gel of Merck grade 9385 in this process under the same conditions saw the concentration of Neutral Red decrease from 100 ppm to between 1.5 and 2.5 ppm within 30 min.
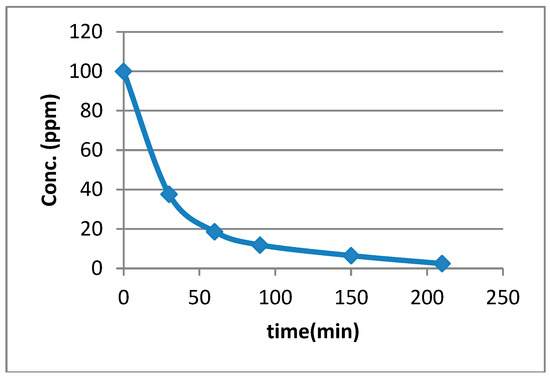
Figure 5.
Adsorption of 100 mL Neutral Red (100 ppm) on 0.5 g silica (coarse, 35–60 mesh) for 3 h 30 min (total adsorption time) at 25 °C.
The max. adsorption capacity of silica gel of Merck grade 9385 (fine, pore size 60 Å, 230–400 mesh) for Neutral Red was found to be 94.7 mg of Neutral Red dye per g of pristine silica gel at 25 °C. Recycling the silica gel did not change the maximum capacity of the silica gel markedly (97.9 mg dye/g of silica gel after 1× recycling of the silica gel). The maximum adsorption capacity of silica gel Aldrich 60742 was found to be 33.6 mg of dye per g of silica gel at 25 °C. Interestingly, in this case, the maximum adsorption capacity of the silica gel increased slightly with increasing temperature (29.6 mg of dye per g of silica gel at 7.0 °C to 36.7 mg of dye per g of silica gel at 41.3 °C).
References
- Huang, C.-H.; Chang, K.-P.; Ou, H.-D.; Chiang, Y.-C.; Wang, C.-F. Adsorption of cationic dyes onto mesoporous silica. Micropor. Mesopor. Mater. 2011, 141, 102–109. [Google Scholar] [CrossRef]
- Cestari, A.R.; Vieira, E.F.; Vieira, G.S.; Costa, L.P.; Tavares, A.M.; Loh, W.; Airoldi, C. The removal of reactive dyes from aqueous solutions using chemically modified mesoporous silica in the presence of anionic surfactant—The temperature dependence and a thermodynamic multivariate analysis. J. Hazard. Mater. 2009, 161, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Karadag, E.; Saraydin, D.; Aydin, F. Removal of Water-Soluble Cationic Dyes with TriSyl Silicas. Turk. J. Chem. 1998, 22, 227–236. [Google Scholar]
- Wahshi, F.; Alqahtani, M.D.; Abdulla, M.; Al-Hemyari, A.; Bufaroosha, M.; Ramachandran, T.; Hamed, F.; Thiemann, T. Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting. Proceedings 2019, 9, 28. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).