Microfluidic Impedance Biosensor Chip with DNA-Based Self-Assembled Monolayers for Label-Free Detection of Cardiac Biomarker Troponin I †
Abstract
:1. Introduction
2. Materials and Methods
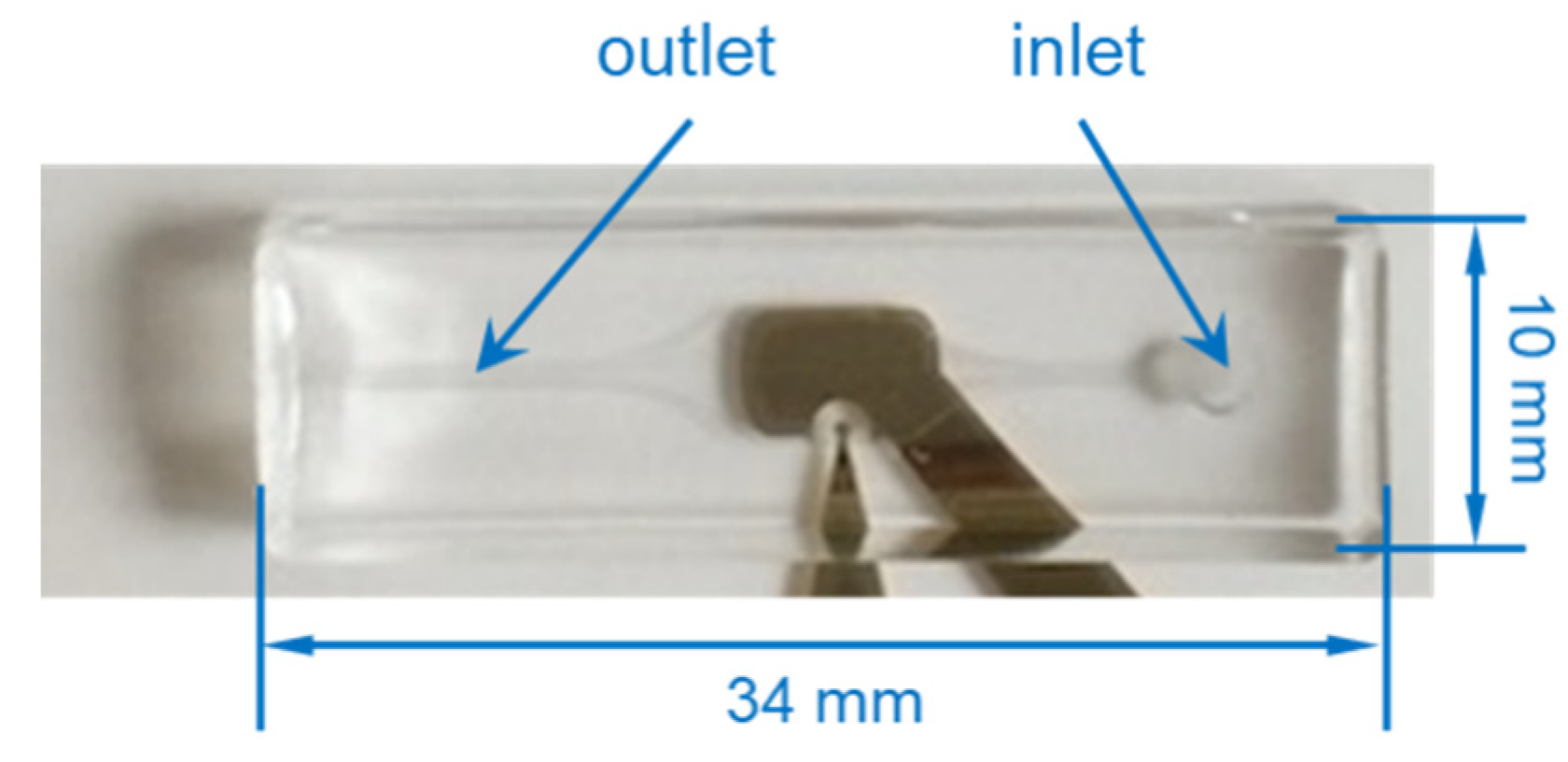
2.1. Fabrication of the Microfluidic Impedance Biosensor Chip
2.1.1. Base Plate and Electrode Sputtering
2.1.2. Microfluidic Channel Fabrication and Connecting
2.2. Surface Functionalization
2.2.1. Application of Thiol-SAMs with Hydrocarbon Spacer, Antibody Immobilization
2.2.2. Application of Thiol-SAMs with DNA Spacer, Antibody Immobilization
2.3. Measurements with the Microfluidic Impedance Biosensor Chip
2.3.1. Measurement Setup
2.3.2. HSA Adsorption for Testing SAMs
2.3.3. HSA Adsorption and Troponin I Assay
3. Results and Discussion
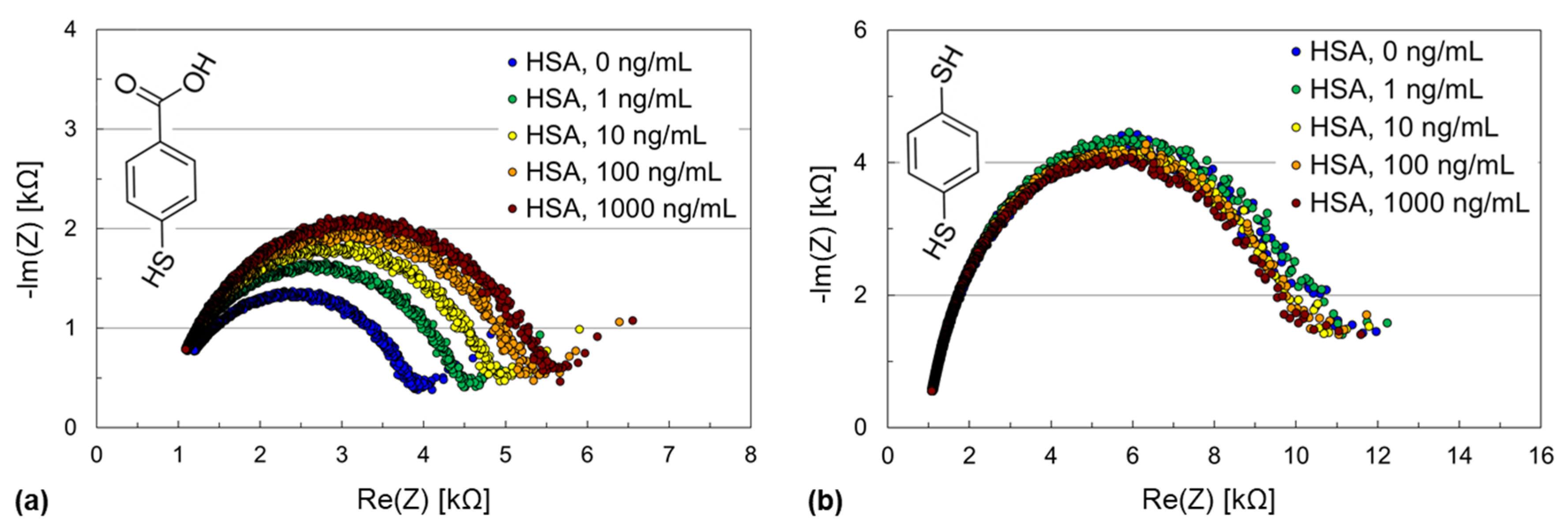
3.1. Troponin I Assay Using Thiol-SAMs Based on Hydrocarbon Spacer
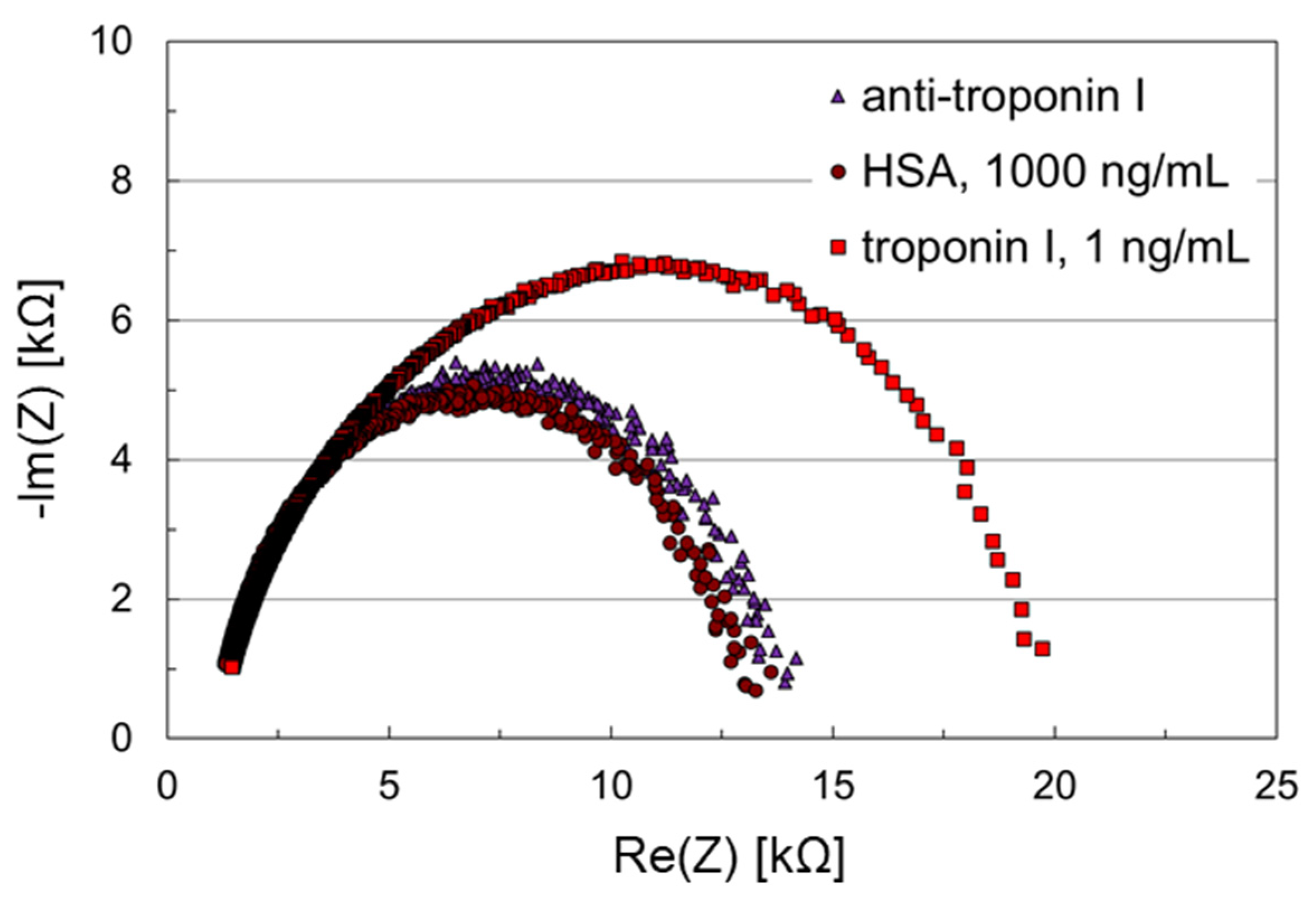
3.2. Troponin I Assay Using Thiol-SAMs Based on DNA Spacer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). WHO Fact Sheet 2017. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 24 September 2020).
- Taylor, C.J.; Ordóñez-Mena, J.M.; Roalfe, A.K.; Lay-Flurrie, S.; Jones, N.R.; Marshall, T.; Hobbs, F.D.R. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: Population based cohort study. BMJ 2019, 364, l223. [Google Scholar] [CrossRef] [PubMed]
- Dörner, K. Klinische Chemie und Hämatologie, 7th ed.; Georg Thieme Verlag: Stuttgart, Germany, 2009. [Google Scholar]
- Luppa, P.B.; Junker, R. (Eds.) Point-of-Care Testing: Principles and Applications, 1st ed.; Springer: Berlin, Germany, 2018. [Google Scholar]
- Gruhl, F.J.; Rapp, B.E.; Länge, K. Biosensors for diagnostic applications. Adv. Biochem. Eng. Biotechnol. 2013, 133, 115–148. [Google Scholar] [PubMed]
- Pires Carneiro, L. Development of an Electrochemical Biosensor Platform and a Suitable Low-Impedance Surface Modification Stategy; KIT Scientific Publishing: Karlsruhe, Germany, 2014. [Google Scholar]
- Leva-Bueno, J.; Peyman, S.A.; Millner, P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020, 209, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Steel, A.B.; Levicky, R.L.; Herne, T.M.; Tarlov, M.J. Immobilization of nucleic acids at solid surfaces: Effect of oligonucleotide length on layer assembly. Biophys. J. 2000, 79, 975–981. [Google Scholar] [CrossRef]
- Rapp, B.E.; Voigt, A.; Dirschka, M.; Länge, K. Deposition of ultrathin parylene C films in the range of 18 nm to 142 nm: Controlling the layer thickness and assessing the closeness of the deposited films. Thin Solid Films 2012, 520, 4884–4888. [Google Scholar] [CrossRef]
- Länge, K.; Gruhl, F.J.; Rapp, M. Surface acoustic wave (SAW) biosensors: Coupling of sensing layers and measurement. Methods Mol. Biol. 2013, 949, 491–505. [Google Scholar] [PubMed]
- Länge, K.; Gruhl, F.J.; Rapp, M. Influence of preparative carboxylation steps on the analyte response of an acoustic biosensor. IEEE Sens. J. 2009, 9, 2033–2034. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsabbagh, K.; Hornung, T.; Voigt, A.; Sadir, S.; Rajabi, T.; Länge, K. Microfluidic Impedance Biosensor Chip with DNA-Based Self-Assembled Monolayers for Label-Free Detection of Cardiac Biomarker Troponin I. Proceedings 2020, 60, 38. https://doi.org/10.3390/IECB2020-07033
Alsabbagh K, Hornung T, Voigt A, Sadir S, Rajabi T, Länge K. Microfluidic Impedance Biosensor Chip with DNA-Based Self-Assembled Monolayers for Label-Free Detection of Cardiac Biomarker Troponin I. Proceedings. 2020; 60(1):38. https://doi.org/10.3390/IECB2020-07033
Chicago/Turabian StyleAlsabbagh, Khaled, Tim Hornung, Achim Voigt, Sahba Sadir, Taleieh Rajabi, and Kerstin Länge. 2020. "Microfluidic Impedance Biosensor Chip with DNA-Based Self-Assembled Monolayers for Label-Free Detection of Cardiac Biomarker Troponin I" Proceedings 60, no. 1: 38. https://doi.org/10.3390/IECB2020-07033




