Preliminary Study on Electrochemical Ion Imprinted Polymeric Film in Sensor Development for Cd(II) Ions Determination in Water †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of Electrosynthesized Ion Imprinted Polymer and Non-Imprinted Polymer
2.4. Cd2+ Ion Sensing
2.5. Experimental Design in Optimization Studies
3. Results and Discussions
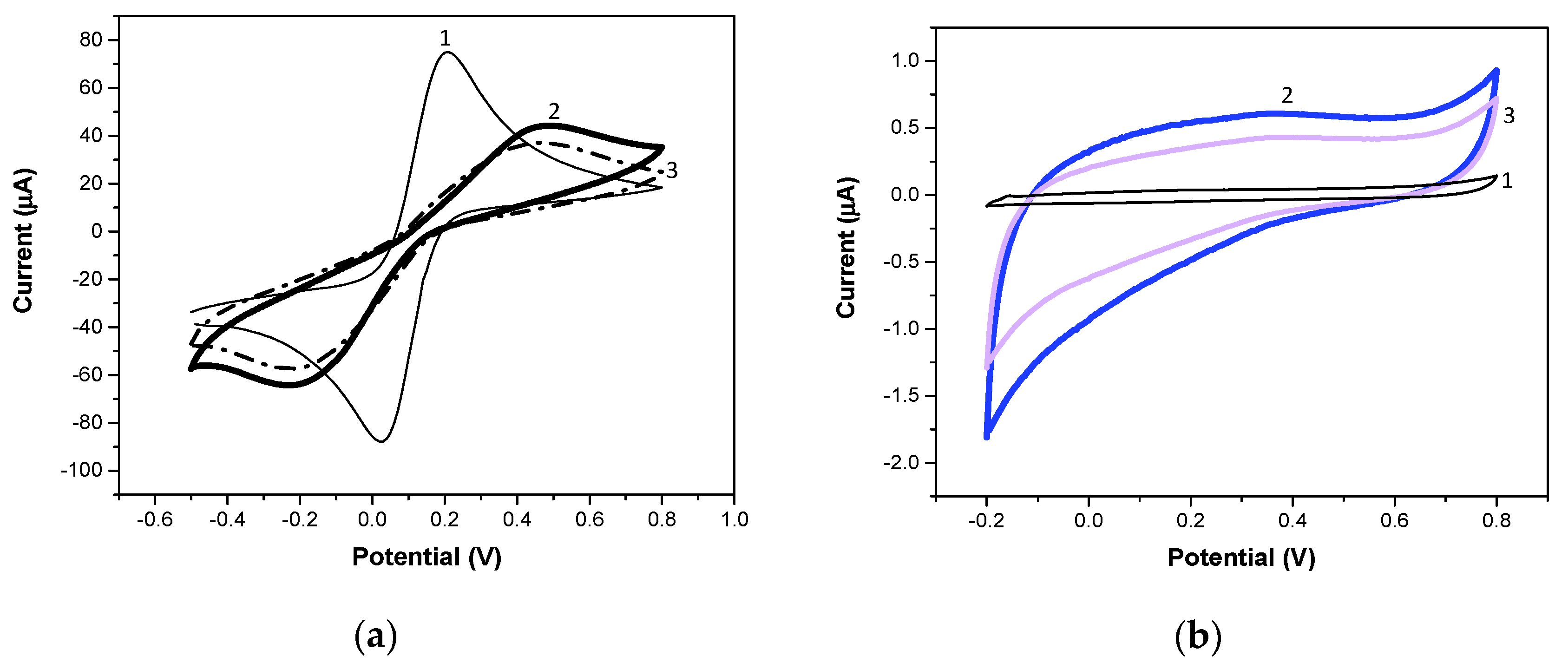
3.1. Preparation of Electrosynthesized IIP and NIP Films
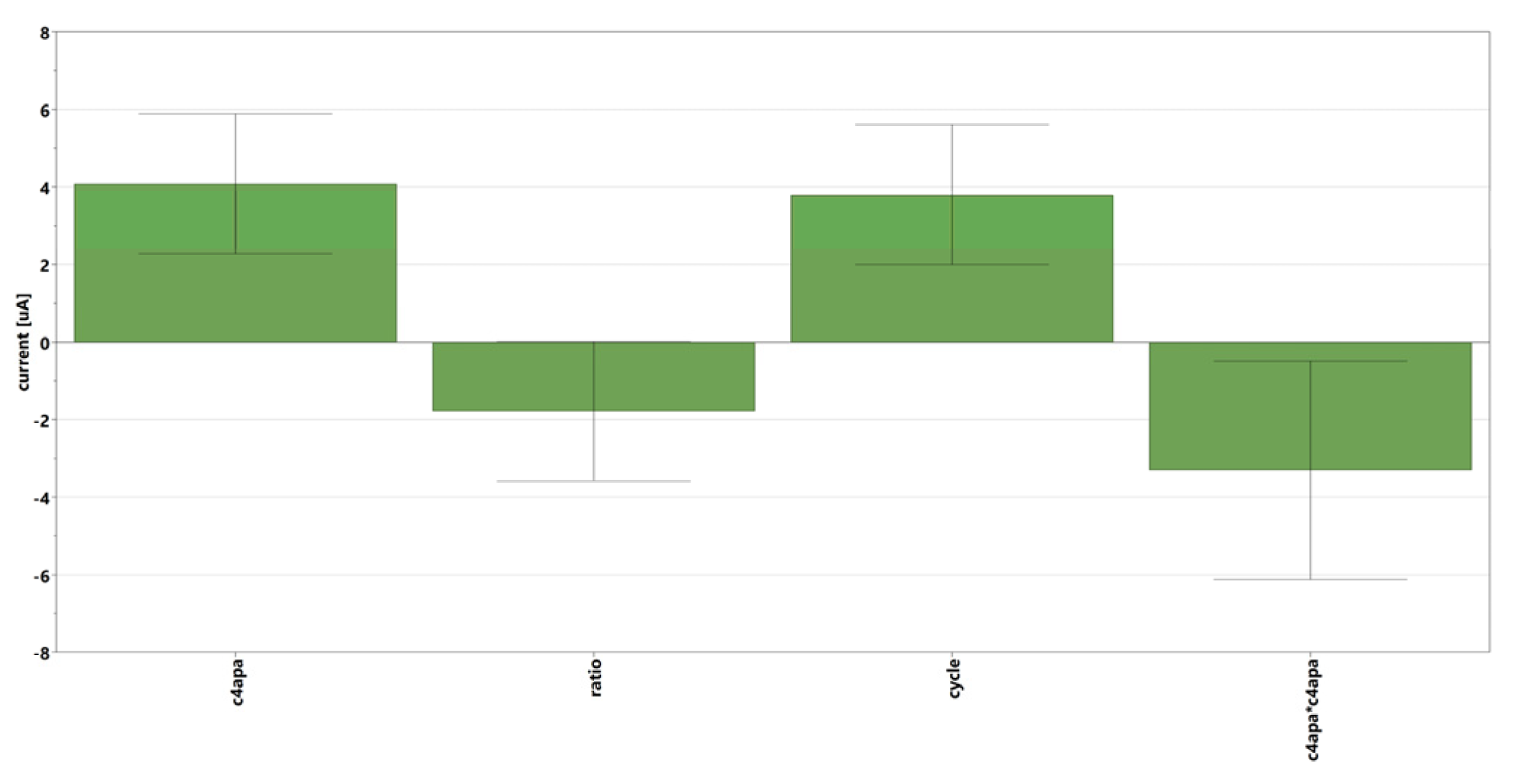
3.2. Optimization of Sensor Performances by Experimental Design
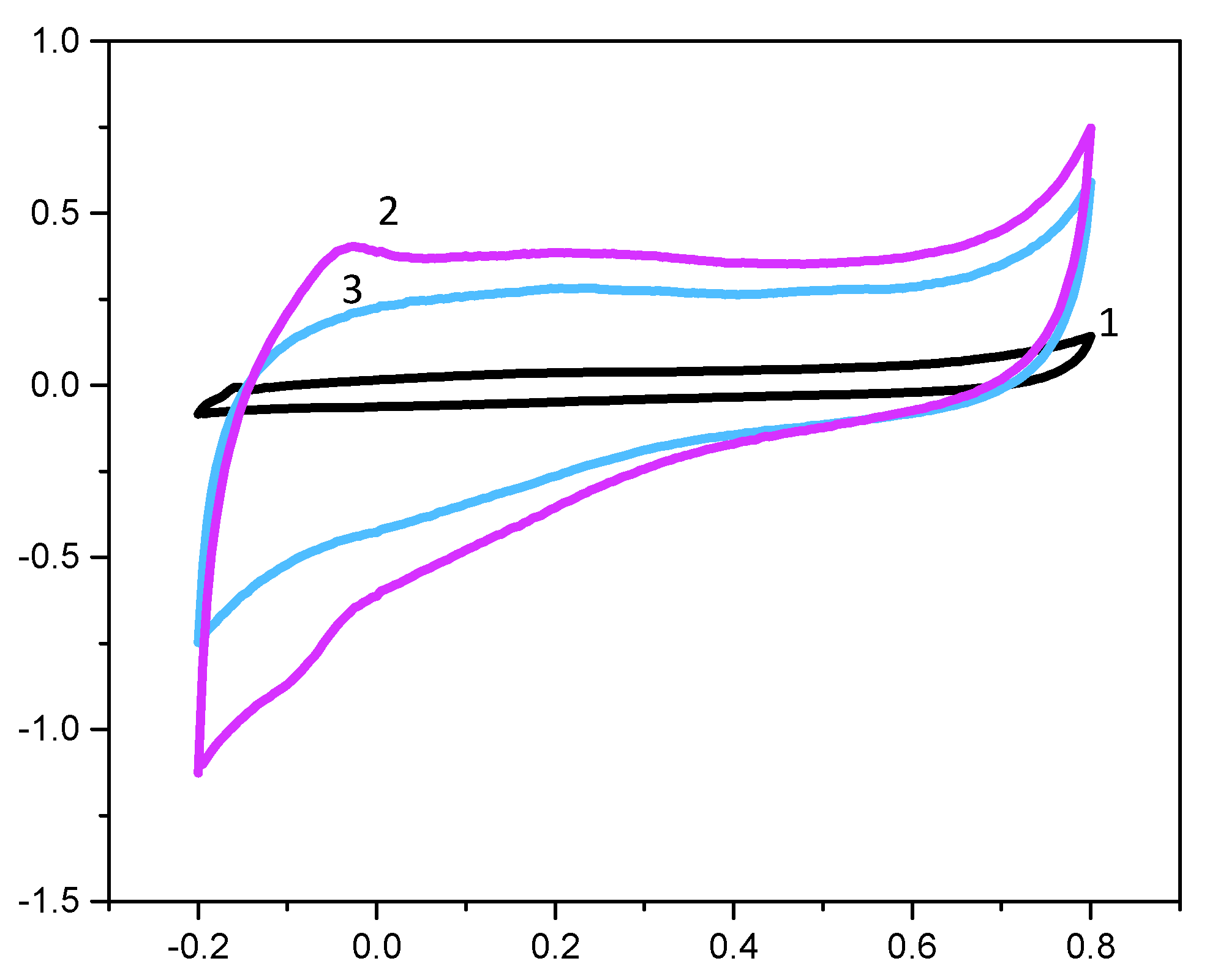
3.3. Electrochemical Characterization of IIP and NIP Films
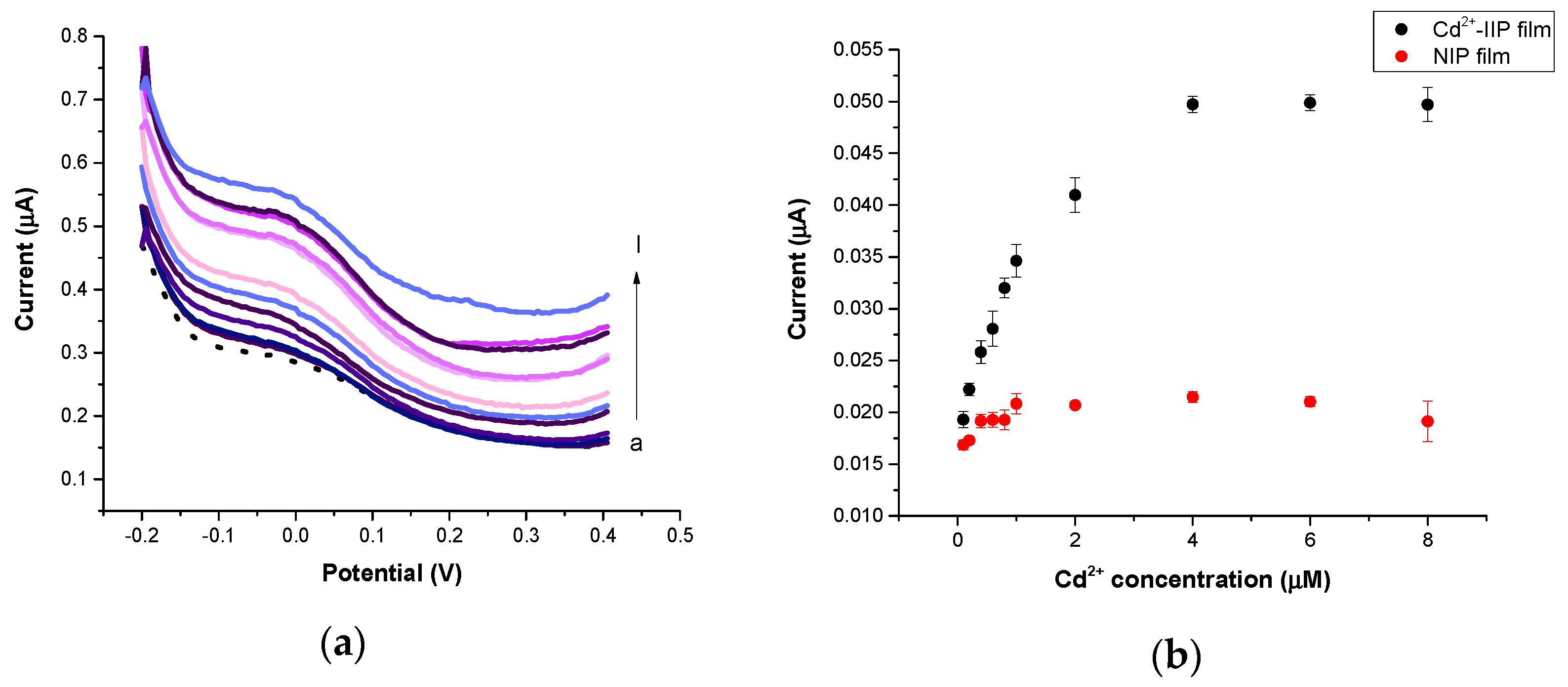
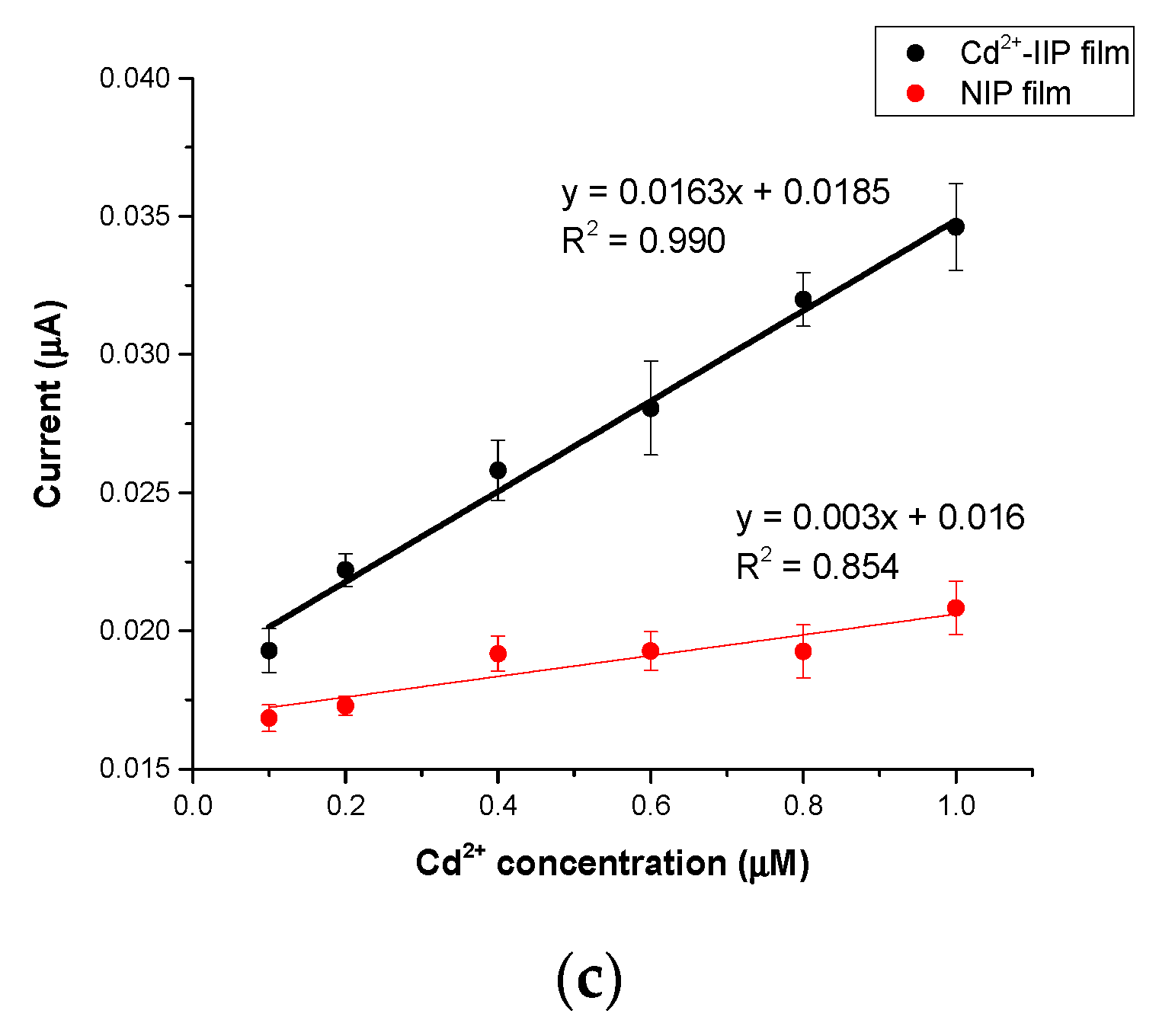
3.4. Electrochemical Performances of IIP and NIP Film
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, A.A.; Ferreira, P.; Santos, A.M.; Cincotto, F.H.; Silva, R.A.B.; Sotomayor, M.D.P.T. A new electrochemical sensor based on eco-friendly chemistry for the simultaneous determination of toxic trace elements. Microchem. J. 2020, 158, 105292. [Google Scholar] [CrossRef]
- Li, N.; Yang, H. Construction of natural polymeric imprinted materials and their applications in water treatment: A review. J. Hazard. Mater. 2021, 403. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, K.K.; Peña-Vázquez, E.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. New adsorbents based on imprinted polymers and composite nanomaterials for arsenic and mercury screening/speciation: A review. Microchem. J. 2020, 156, 104886. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Pietrzyk-Le, A.; D’Souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kandasubramanian, B. Molecularly Imprinted Polymers for Selective Recognition and Extraction of Heavy Metal Ions and Toxic Dyes. J. Chem. Eng. Data 2020, 65, 396–418. [Google Scholar] [CrossRef]
- Di Masi, S.; Pennetta, A.; Guerreiro, A.; Canfarotta, F.; Egidio, G.; Benedetto, D.; Malitesta, C. Sensor based on electrosynthesised imprinted polymeric film for rapid and trace detection of copper (II) ions. Sensors Actuators B. Chem. 2020, 307, 127648. [Google Scholar] [CrossRef]
- Pimenta, T.C.; Santos, C.d.C.; Thomasini, R.L.; Ferreira, L.F. Impedimetric immunosensor for dengue diagnosis using graphite screen-printed electrodes coated with poly(4-aminophenylacetic acid). Biomed. Microdevices 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Variable | Low | High |
|---|---|---|
| Monomer concentration (X1) | 0.5 | 5 |
| Rate Cd2+/monomer (X2) | 1 | 3 |
| Number of CV cycles (X3) | 10 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, S.D.; Pennetta, A.; Malitesta, C. Preliminary Study on Electrochemical Ion Imprinted Polymeric Film in Sensor Development for Cd(II) Ions Determination in Water. Proceedings 2020, 60, 39. https://doi.org/10.3390/IECB2020-07037
Masi SD, Pennetta A, Malitesta C. Preliminary Study on Electrochemical Ion Imprinted Polymeric Film in Sensor Development for Cd(II) Ions Determination in Water. Proceedings. 2020; 60(1):39. https://doi.org/10.3390/IECB2020-07037
Chicago/Turabian StyleMasi, Sabrina Di, Antonio Pennetta, and Cosimino Malitesta. 2020. "Preliminary Study on Electrochemical Ion Imprinted Polymeric Film in Sensor Development for Cd(II) Ions Determination in Water" Proceedings 60, no. 1: 39. https://doi.org/10.3390/IECB2020-07037
APA StyleMasi, S. D., Pennetta, A., & Malitesta, C. (2020). Preliminary Study on Electrochemical Ion Imprinted Polymeric Film in Sensor Development for Cd(II) Ions Determination in Water. Proceedings, 60(1), 39. https://doi.org/10.3390/IECB2020-07037







