Ceramic Soil Microbial Fuel Cells Sensors for Early Detection of Eutrophication †
Abstract
:1. Introduction
2. Materials and Methods
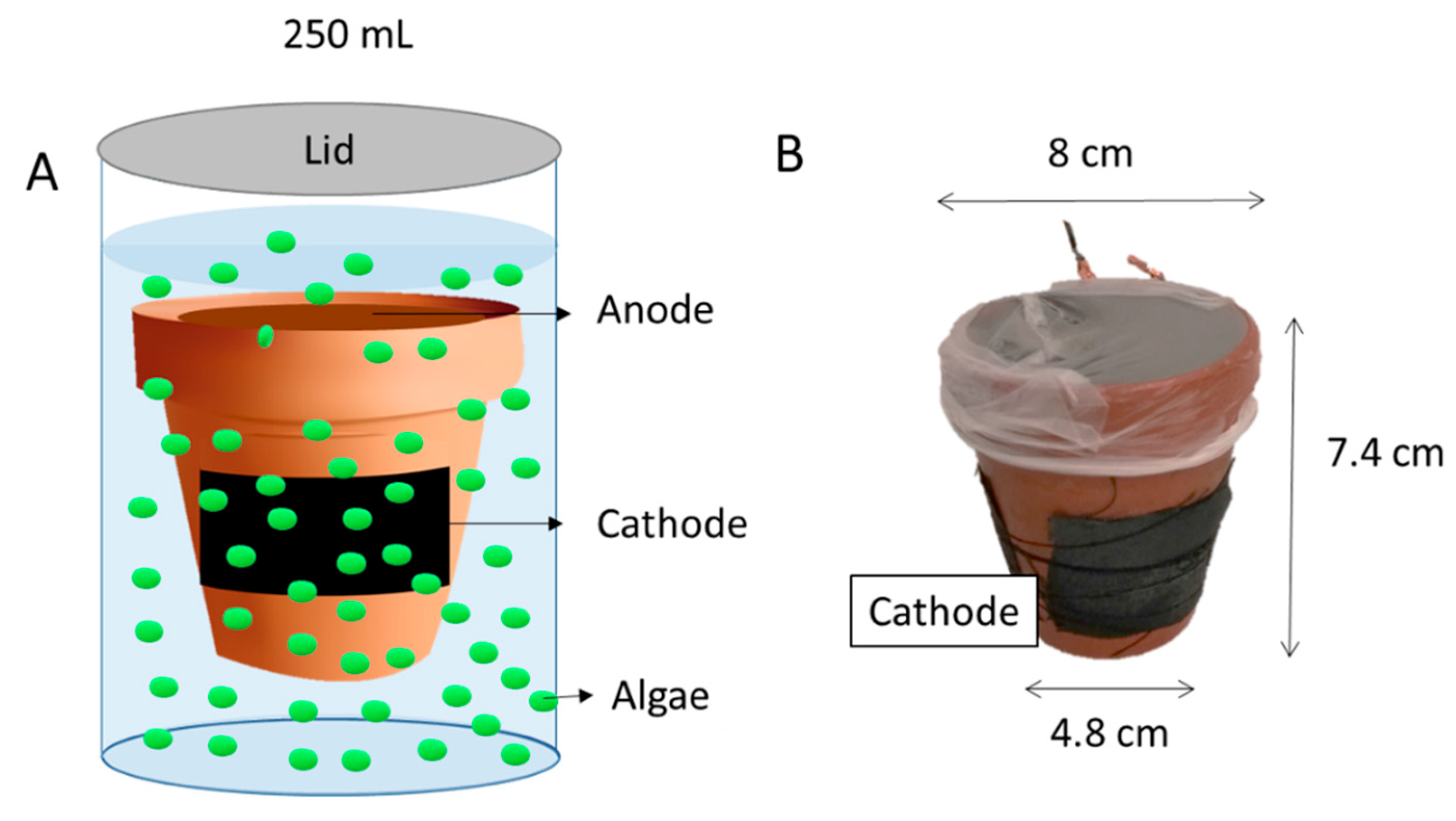
2.1. Materials
2.2. Operation of the CSMFC in Eutrophic Water
2.3. Electrochemical Characterisation
2.4. Influence of Factors on the CSMFC Response
3. Results and Discussion
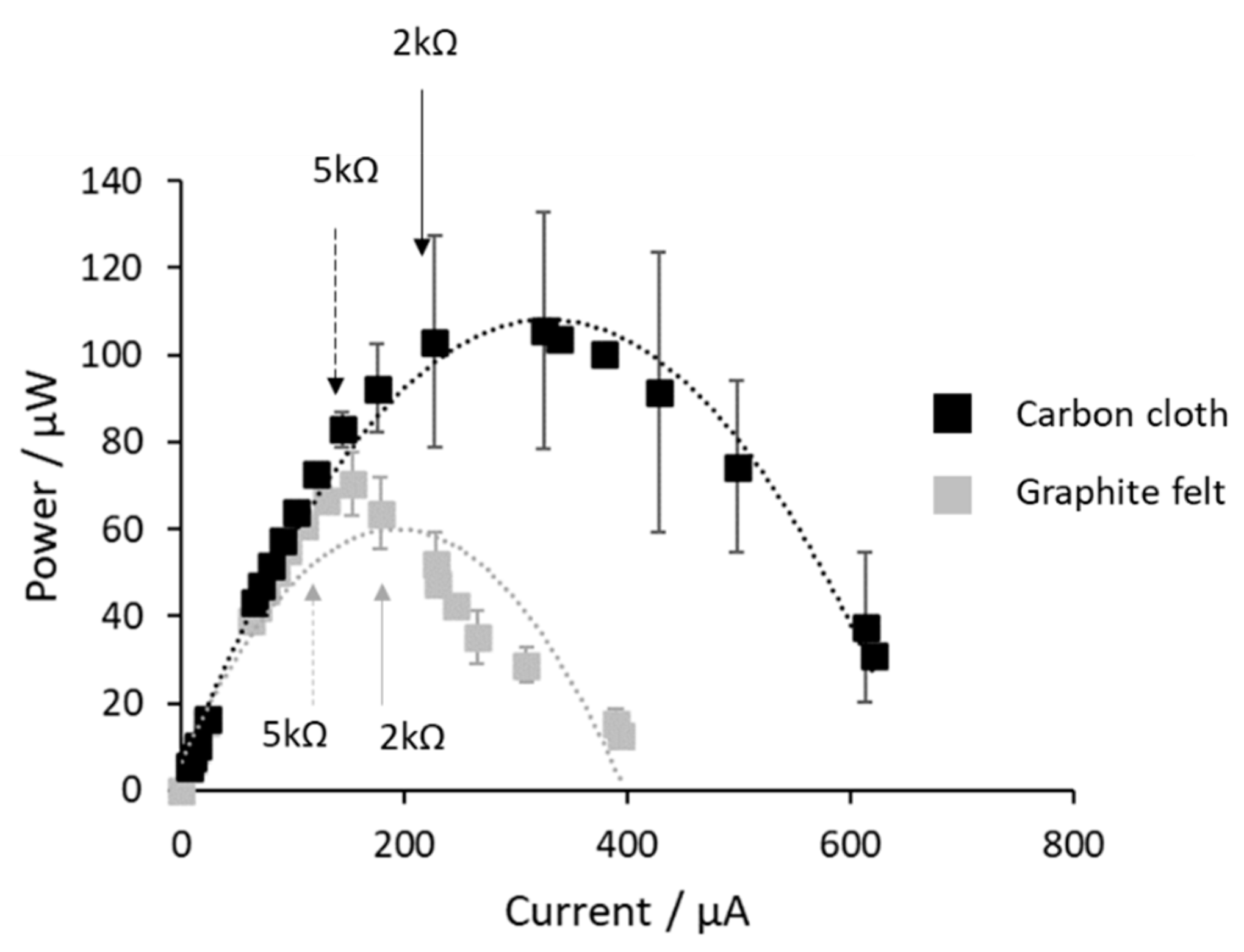
3.1. Enrichment and Operation of CSMFC as DO Sensor in Eutrophic Waters
3.2. Calibration of the CSMFC Sensor
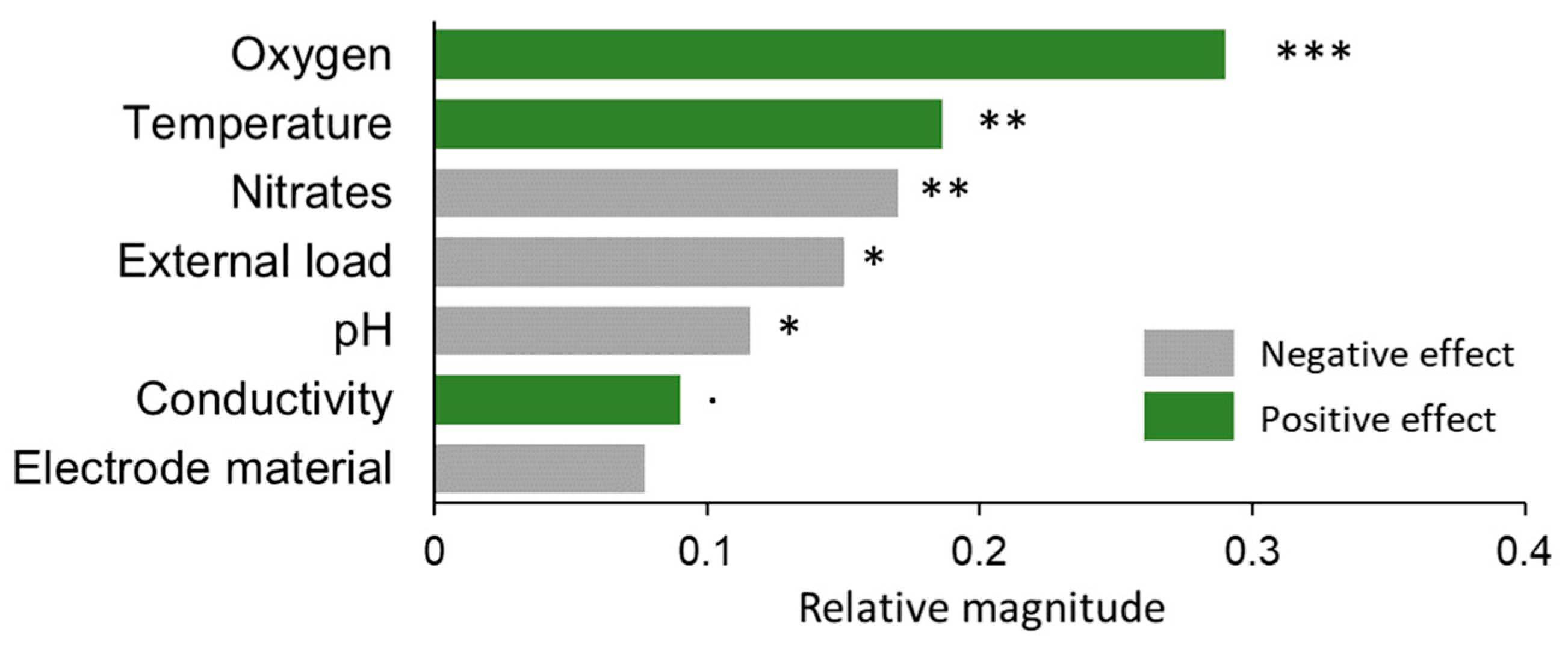
3.3. Evaluation of Relevant Factors on CSMFC Performance
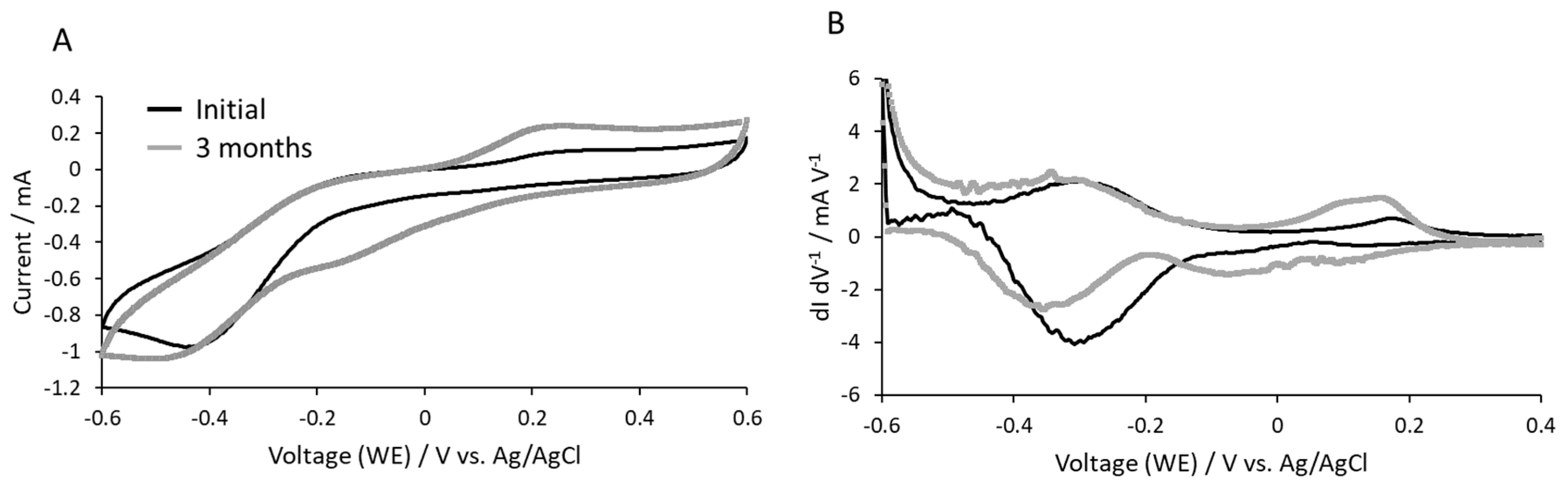
3.4. Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- EEA. Nutrient Enrichment and Eutrophication in Europe’s Seas; EEA: Copenhagen, Denmark, 2019. [Google Scholar]
- Lürling, M.; Mucci, M. Mitigating eutrophication nuisance: In-lake measures are becoming inevitable in eutrophic waters in the Netherlands. Hydrobiologia 2020, 847, 4447–4467. [Google Scholar] [CrossRef]
- NOWPAP. Eutrophication Monitoring Guidelines by Remote Sensing for the NOWPAP Region; CEARAC Rep. UNEP Reg. Sea p. 62s; NOWPAP: Toyama, Japan, 2007. [Google Scholar]
- Dai, Z.; Xu, Z.; Wang, T.; Fan, Y.; Liu, Y.; Yu, R.; Zhu, G.; Lu, X.; Li, B. In-situ oil presence sensor using simple-structured upward open-channel microbial fuel cell (UOC-MFC). Biosens. Bioelectron. X 2019, 1, 100014. [Google Scholar] [CrossRef]
- Chouler, J.; di Lorenzo, M. Water quality monitoring in developing countries; Can microbial fuel cells be the answer? Biosensors 2015, 5, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Watanabe, T.; Maki, H.; Kanaya, G.; Higashi, H.; Syutsubo, K. Operation of sediment microbial fuel cells in Tokyo Bay, an extremely eutrophicated coastal sea. Bioresour. Technol. Rep. 2019, 6, 39–45. [Google Scholar] [CrossRef]
- Olias, L.G.; Rodr, A.; Cameron, P.J.; di Lorenzo, M. A soil microbial fuel cell-based biosensor for dissolved oxygen. Electrochim. Acta 2020, 362, 137108. [Google Scholar] [CrossRef]
- Dunn, K.G. Process Improvement Using Data. Available online: http://learnche.org/pid (accessed on 21 October 2019).
- McCrady, M.H. Standard methods for the examination of water and waste-water. Am. J. Public Health Nations Health 2008, 56, 684–684. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Photoautotrophic microalgae Scenedesmus obliquus attached on a cathode as oxygen producers for microbial fuel cell (MFC) operation. Int. J. Hydrogen Energy 2014, 39, 10275–10283. [Google Scholar] [CrossRef]
- Martinez, S.M.; di Lorenzo, M. Electricity generation from untreated fresh digestate with a cost-effective array of floating microbial fuel cells. Chem. Eng. Sci. 2019, 198, 108–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Biosens. Bioelectron. 2012, 38, 189–194. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Self-powered, autonomous Biological Oxygen Demand biosensor for online water quality monitoring. Sens. Actuators B Chem. 2017, 244, 815–822. [Google Scholar] [CrossRef]
- del Campo, A.G.; Cañizares, P.; Rodrigo, M.A.; Fernández, F.J.; Lobato, J. Microbial fuel cell with an algae-assisted cathode : A preliminary assessment. J. Power Sources 2013, 242, 638–645. [Google Scholar] [CrossRef]
- Lobato, J.; del Campo, A.G.; Fernández, F.J.; Cañizares, P.; Rodrigo, M.A. Lagooning microbial fuel cells: A first approach by coupling electricity-producing microorganisms and algae. Appl. Energy 2013, 110, 220–226. [Google Scholar] [CrossRef]
- He, Z.; Kan, J.; Mansfeld, F.; Angenent, L.T.; Nealson, K.H. Self-sustained phototrophic microbial fuel cells based on the synergistic cooperation between photosynthetic microorganisms and heterotrophic bacteria. Environ. Sci. Technol. 2009, 43, 1648–1654. [Google Scholar] [CrossRef]
- Wu, X.Y.; Song, T.S.; Zhu, X.J.; Wei, P.; Zhou, C.C. Construction and operation of microbial fuel cell with Chlorella vulgaris biocathode for electricity generation. Appl. Biochem. Biotechnol. 2013, 171, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, S.; Anam, M.; Saeed, S.; Ali, N. Environmental Technology Reviews Electricigens: Source, enrichment and limitations Electricigens: Source, enrichment and limitations. Environ. Technol. Rev. 2017, 6, 117–134. [Google Scholar] [CrossRef]
- Song, X.; Wang, W.; Cao, X.; Wang, Y.; Zou, L.; Ge, X.; Zhao, Y.; Si, Z.; Wang, Y. Chlorella vulgaris on the cathode promoted the performance of sediment microbial fuel cells for electrogenesis and pollutant removal. Sci. Total Environ. 2020, 728, 138011. [Google Scholar] [CrossRef]
- Song, N.; Yan, Z.; Xu, H.; Yao, Z.; Wang, C.; Chen, M.; Zhao, Z.; Peng, Z.; Wang, C.; Jiang, H.-L. Development of a sediment microbial fuel cell-based biosensor for simultaneous online monitoring of dissolved oxygen concentrations along various depths in lake water Science of the Total Environment Development of a sediment microbial fuel cell-based bio. Sci. Total Environ. 2019, 673, 272–280. [Google Scholar] [CrossRef]
- Olias, L.G.; Cameron, P.J.; di Lorenzo, M. Effect of Electrode Properties on the Performance of a Photosynthetic Microbial Fuel Cell for Atrazine Detection. Front. Energy Res. 2019, 7, 1–11. [Google Scholar]
- Mao, J. Oxygen Reduction Reaction. Electrocatalysis 2019, 1, 8785–8789. [Google Scholar]
- Wang, C.-T.; Huang, Y.-S.; Sangeetha, T.; Chen, Y.-M.; Chong, W.-T.; Ong, H.-C.; Zhao, F.; Yan, W.-M. Novel bufferless photosynthetic microbial fuel cell (PMFCs) for enhanced electrochemical performance. Bioresour. Technol. 2018, 255, 83–87. [Google Scholar] [CrossRef]
- Fu, L.; You, S.J.; Yang, F.L.; Gao, M.M.; Fang, X.H.; Zhang, G.Q. Synthesis of hydrogen peroxide inmicrobial fuel cell. J. Chem. Technol. Biotechnol. 2010, 85, 715–719. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Srikanth, S.; Chiranjeevi, P.; Arora, S.; Chandra, R. Algal biocathode for in situ terminal electron acceptor (TEA) production: Synergetic association of bacteria-microalgae metabolism for the functioning of biofuel cell. Bioresour. Technol. 2014, 166, 566–574. [Google Scholar] [CrossRef] [PubMed]
- YSI. The Dissolved Oxygen Handbook; YSI: Yellow Springs, OH, USA, 2009; p. 43. [Google Scholar]
- Commault, A.S.; Lear, G.; Novis, P.; Weld, R.J. Photosynthetic biocathode enhances the power output of a sediment-type microbial fuel cell. New Z. J. Bot. 2014, 52, 48–59. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Liao, C.; Wu, J.; Zhou, L.; Li, T.; Du, Q.; An, J.; Wang, X. Optimal set of electrode potential enhances the toxicity response of biocathode to formaldehyde. Sci. Total Environ. 2018, 644, 1485–1492. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Weigele, P.R. A terracotta bio-battery. Bioresour. Technol. 2012, 116, 86–91. [Google Scholar] [CrossRef]
- Bardarov, I.; Mitov, M.; Ivanova, D.; Hubenova, Y. Light-dependent processes on the cathode enhance the electrical outputs of sediment microbial fuel cells. Bioelectrochemistry 2018, 122, 1–10. [Google Scholar] [CrossRef]
- Strycharz-Glaven, S.M.; Glaven, R.H.; Wang, Z.; Zhou, J.; Vora, G.J.; Tender, L.M. Electrochemical investigation of a microbial solar cell reveals a nonphotosynthetic biocathode catalyst. Appl. Environ. Microbiol. 2013, 79, 3933–3942. [Google Scholar] [CrossRef]
- González-García, J.; Bonete, P.; Expósito, E.; Montiel, V.; Aldaz, A.; Torregrosa-Maciá, R. Characterization of a carbon felt electrode: Structural and physical properties. J. Mater. Chem. 1999, 9, 419–426. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Nesaraj, A.S.; Noel, M.; James, E.J. Effect of chemically treated / untreated carbon cloth: Potential use as electrode materialsin the capacitive deionization process of desalination of aqueous salt solution. J. Electrochem. Sci. Technol. 2015, 6, 139–145. [Google Scholar] [CrossRef]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An overview of electron acceptors in microbial fuel cells. Front. Microbiol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Labelle, E.; Bond, D.R. Cyclic voltammetry of electrode-attached bacteria. In Bio-Electrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application; Wiley-VCH Verlag: Weinheim, Germany, 2005; pp. 1–15. [Google Scholar]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6–11. [Google Scholar] [CrossRef]
- Struyk, Z.; Sposito, G. Redox properties of humic acids. Geoderma 2001, 102, 329–346. [Google Scholar] [CrossRef]



| Factors | Level | |
|---|---|---|
| + | − | |
| Temperature, T/°C | 30 | 10 |
| Dissolved oxygen, DO/mg L−1 | 10 | 1 |
| pH/- | 9 | 5 |
| Conductivity, C/µS cm−1 | 3000 | 150 |
| Electrode material, M | GF | CC |
| Rext/Ω | 5000 | 2000 |
| NO3/mg L−1 | 10 | 1 |
| Run | M | C | DO | NO3 = M × C | pH = M × DO | T = C × DO | Rext = M × C × DO | V1 | V2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CC | 300 | 1 | 10 | 9 | 30 | 2000 | 1.06 | 0.60 |
| 2 | CF | 300 | 1 | 1 | 5 | 30 | 5000 | 0.96 | 0.95 |
| 3 | CC | 3000 | 1 | 1 | 9 | 10 | 5000 | 0.70 | 0.99 |
| 4 | CF | 3000 | 1 | 10 | 5 | 10 | 2000 | 0.73 | 0.74 |
| 5 | CC | 300 | 10 | 10 | 5 | 10 | 5000 | 0.88 | 1.05 |
| 6 | CF | 300 | 10 | 1 | 9 | 10 | 2000 | 1.24 | 1.01 |
| 7 | CC | 3000 | 10 | 1 | 5 | 30 | 2000 | 1.47 | 2.13 |
| 8 | CF | 3000 | 10 | 10 | 9 | 30 | 5000 | 1.08 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olias, L.G.; Otero, A.R.; Cameron, P.J.; Lorenzo, M.D. Ceramic Soil Microbial Fuel Cells Sensors for Early Detection of Eutrophication. Proceedings 2020, 60, 64. https://doi.org/10.3390/IECB2020-07039
Olias LG, Otero AR, Cameron PJ, Lorenzo MD. Ceramic Soil Microbial Fuel Cells Sensors for Early Detection of Eutrophication. Proceedings. 2020; 60(1):64. https://doi.org/10.3390/IECB2020-07039
Chicago/Turabian StyleOlias, Lola Gonzalez, Alba Rodríguez Otero, Petra J. Cameron, and Mirella Di Lorenzo. 2020. "Ceramic Soil Microbial Fuel Cells Sensors for Early Detection of Eutrophication" Proceedings 60, no. 1: 64. https://doi.org/10.3390/IECB2020-07039
APA StyleOlias, L. G., Otero, A. R., Cameron, P. J., & Lorenzo, M. D. (2020). Ceramic Soil Microbial Fuel Cells Sensors for Early Detection of Eutrophication. Proceedings, 60(1), 64. https://doi.org/10.3390/IECB2020-07039





