Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage †
Abstract
:1. Introduction
2. Experimental Methods
3. Characterization
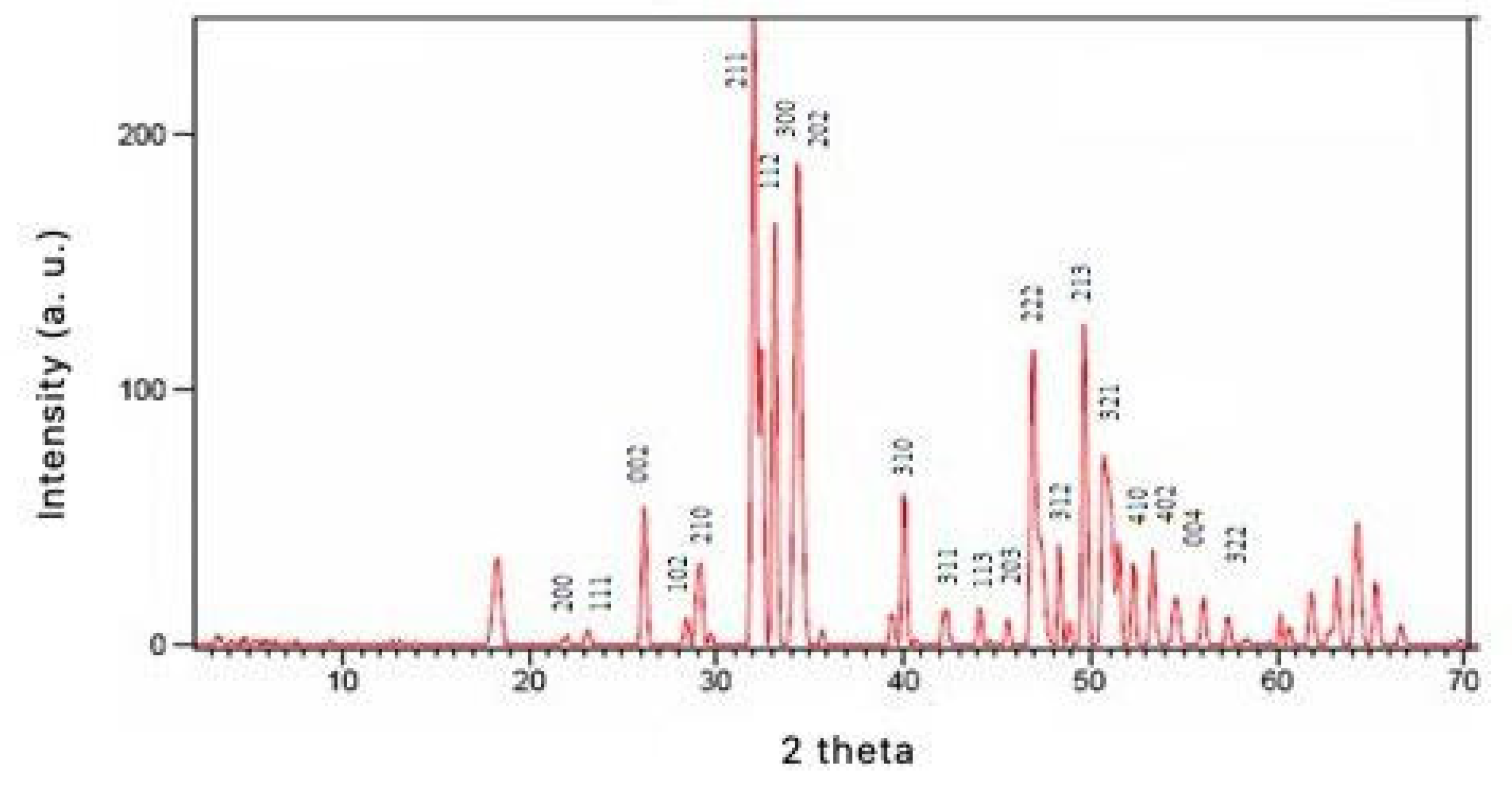
3.1. X-ray Diffraction
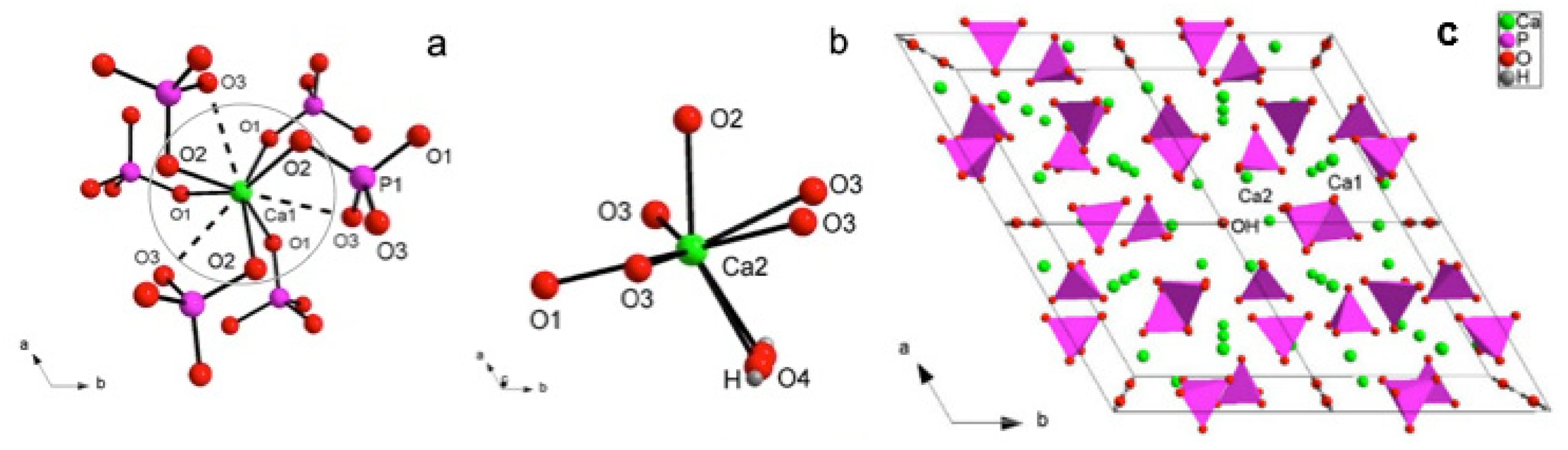
3.2. X-ray Crystal Structure
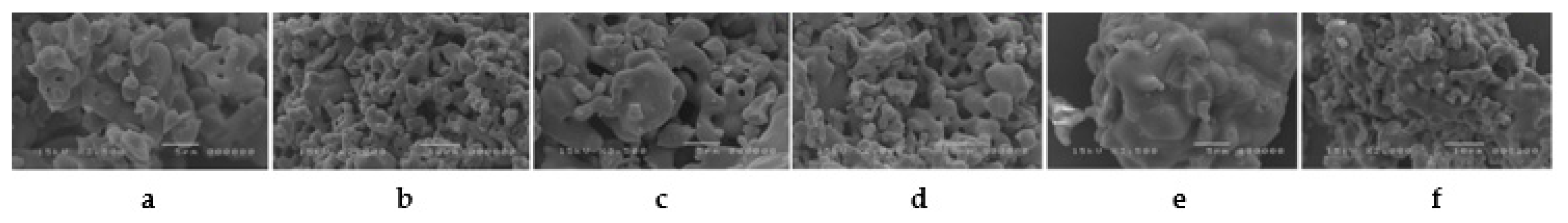
3.3. SEM Microscopy
3.4. FTIR Spectroscopy
3.5. SAXS/WAXS
3.6. Biological Evaluation of the Antimicrobial Properties of Obtained Nanophases
3.7. LIBS
3.8. Carbonatic Substrate Treatment
3.9. Acid Attack Preliminary Test
Funding
Acknowledgments
References
- Pesce, C.; Moretto, L.M.; Orsega, E.F.; Pesce, G.L.; Corradi, M.; Weber, J. Effectiveness and Compatibility of a Novel Sustainable Method for Stone Consolidation Based on Di-Ammonium Phosphate and Calcium-Based Nanomaterials. Materials 2019, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Estebanez, M.; Ortega-Morales, B.O.; Chan-Bacab, M.; Granados-Echegoyen, C.; Camacho-Chab, J.C.; Pereanez-Sacarias, J.E.; Gaylarde, C. Antimicrobial engineered nanoparticles in the built cultural heritage context and their ecotoxicological impact on animals and plants: A brief review. Herit. Sci. 2018, 6, 52. [Google Scholar] [CrossRef]
- El Bakkari, M.; Bindiganavile, V.; Boluk, Y. Facile Synthesis of Calcium Hydroxide Nanoparticles onto TEMPO-Oxidized Cellulose Nanofibers for Heritage Conservation. ACS Omega 2019, 4, 20606–20611. [Google Scholar] [CrossRef]
- Dida, B.; Siliqi, D.; Baldassarre, F.; Karaj, D.; Hasimi, A.; Kasemi, V.; Nika, V.; Vozga, I. Nanomaterialet për Konservimin e Trashëgimisë Kulturore; SHLBSH: Tirana, Albania, 2020; ISBN 978-99943-2-468-2. [Google Scholar]
- Rakovan, J.R.; Pasteris, J.D. A technological gem: Materials, Medical, and Environmental Mineralogy of Apatite. Elements 2015, 11, 195–200. [Google Scholar] [CrossRef]
- Baldassarre, F.; Altomare, A.; Corriero, N.; Mesto, E.; Lacalamita, M.; Bruno, G.; Sacchetti, A.; Dida, B.; Karaj, D.; Della Ventura, G.D.; et al. Crystal Chemistry and Luminescence Properties of Eu-Doped Polycrystalline Hydroxyapatite Synthesized by Chemical Precipitation at Room Temperature. Crystals 2020, 10, 250. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E.; Scherer, G.W. Hydroxyapatite Coatings for Marble Protection: Optimization of Calcite Covering and Acid Resistance. Appl. Surf. Sci. 2016, 368, 241–257. [Google Scholar] [CrossRef]
- Angenault, J.; Couturier, J.C.; Souron, J.P.; Siliqi, D.; Quarton, M. The martensitic nature of the transition monoclinic ⇌ rhombohedral of LiSn2(PO4)3. J. Mater. Sci. Lett. 1992, 11, 1705–1707. [Google Scholar] [CrossRef]
- Capitelli, F.; Harcharras, M.; Assaaoudi, H.; Ennaciri, A.; Moliterni, A.G.G.; Bertolasi, V. Crystal structure of new hexahydrate dicobalt pyrophosphate Co2P2O7·6H2O: comparison with Co2P2O7·2H2O, α-, β- and γ- Co2P2O7. Z. Kristallogr. 2003, 218, 345–350. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- Glatter, O.; Kratky, O. Small-Angle X-Ray Scattering; Academic: London, UK, 1982. [Google Scholar]
- Altamura, D.; Lassandro, R.; Vittoria, F.A.; De Caro, L.; Siliqi, D.; Ladisa, M.; Giannini, C. X-ray microimaging laboratory (XMI-LAB). J. Appl. Crystallogr. 2012, 45, 869–873. [Google Scholar] [CrossRef]
- Resmin, C.M.; Dalpasquale, M.; Vielmo, N.I.C.; Mariani, F.Q.; Villalba, J.C.; Anaissi, F.J.; Caetano, M.M.; Tusi, M.M. Study of physico-chemical properties and in vitro antimicrobial activity of hydroxyapatites obtained from bone calcination. Prog. Biomater. 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Phatai, P.; Futalan, C.M.; Kamonwannasit, S.; Khemthong, P. Structural characterization and antibacterial activity of hydroxyapatite synthesized via sol-gel method using glutinous rice as a template. J. Sol-Gel. Sci. Technol. 2019, 89, 764–775. [Google Scholar] [CrossRef]
- Senesi, G.S. Laser-Induced Breakdown Spectroscopy (LIBS) applied to terrestrial and extraterrestrial analogue geomaterials with emphasis to minerals and rocks. Earth-Sci. Rev. 2014, 139, 231–267. [Google Scholar] [CrossRef]
- Senesi, G.S.; Capitelli, F. Compositional, mineralogical and structural investigation of meteorites by XRD and LIBS. In Hypersonic Meteoroid Entry Physics; Colonna, G., Capitelli, M., Laricchiuta, A., Eds.; IOP Series in Plasma Physics; IOP Publishing: Bristol, UK, 2019; pp. 5-1–5-39. [Google Scholar]
- Anglos, D. Laser-induced breakdown spectroscopy in heritage science. Phys. Sci. Rev. 2019, 4, 20180005. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behavior and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P. Rietveld refinement of the crystallographic structure of human dental enamel apatites. Am. Mineral. 1999, 84, 1406–1414. [Google Scholar] [CrossRef]
- Rossi, M.; Ghiara, M.R.; Chita, G.; Capitelli, F. Crystal-chemical and structural characterization of fluorapatites in ejecta from Somma-Vesuvius volcanic complex. Am. Mineral. 2011, 96, 1828–1837. [Google Scholar] [CrossRef]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Sol. State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Kong, L.B.; Ma, J.; Boey, F. Nanosized hydroxyapatite powders derived from coprecipitation process. J. Mater. Sci. 2002, 37, 1131–1134. [Google Scholar] [CrossRef]
- Skorokhod, V.V.; Solonin, S.M.; Dubok, V.A.; Kolomiets, L.L.; Permyakova, T.V.; Shinkaruk, A.V.; Solonin, S.M.; Dubok, V.A.; Kolomiets, L.L.; Permyakova, T.V.; et al. Decomposition activation of hydroxyapatite in contact with β-tricalcium phosphate. Powder Metall. Met. C+ 2010, 49, 5–6. [Google Scholar] [CrossRef]
- El Khouri, A.; Elaatmani, M.; Della Ventura, G.; Sodo, A.; Rizzi, R.; Rossi, M.; Capitelli, F. Synthesis, structure refinement and vibrational spectroscopy of new rare-earth tricalcium phosphates Ca9RE(PO4)7 (RE = La, Pr, Nd, Eu, Gd, Dy, Tm, Yb). Ceram. Int. 2017, 43, 15645–15653. [Google Scholar] [CrossRef]
- Capitelli, F.; Rossi, M.; El Khouri, A.; Elaatmani, M.; Corriero, N.; Sodo, A.; Della Ventura, G. Synthesis, structural model and vibrational spectroscopy of lutetium tricalcium phosphate Ca9Lu(PO4)7. J. Rare Earth 2018, 36, 1162–1168. [Google Scholar] [CrossRef]
- Altomare, A.; Rizzi, R.; Rossi, M.; El Khouri, A.; Elaatmani, M.; Paterlini, V.; Della Ventura, G.; Capitelli, F. New Ca2.90(Me2+)0.10(PO4)2 β-tricalcium Phosphates with Me2+ = Mn, Ni, Cu: Synthesis, Crystal-Chemistry, and Luminescence Properties. Crystals 2019, 9, 288. [Google Scholar] [CrossRef]
- Paterlini, V.; Bettinelli, M.; Rizzi, R.; El Khouri, A.; Rossi, M.; Della Ventura, G.; Capitelli, F. Characterization and Luminescence of Eu3+- and Gd3+-Doped Hydroxyapatite Ca10(PO4)6(OH)2. Crystals 2020, 10, 806. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Jastrzębski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared spectroscopy of different phosphates structures. Spectrochim. Acta A 2011, 79, 722–727. [Google Scholar] [CrossRef]
- El Khouri, A.; Zegzouti, A.; Elaatmani, M.; Capitelli, F. Bismuth-substituted hydroxyapatite ceramics synthesis: Morphological, structural, vibrational and dielectric properties. Inorg. Chem. Comm. 2019, 110, 107568. [Google Scholar] [CrossRef]
- Iconaru, S.-L.; Motelica-Heino, M.; Predoi, D. Study on Europium-Doped Hydroxyapatite Nanoparticles by Fourier Transform Infrared Spectroscopy and Their Antimicrobial Properties. J. Spectrosc. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553. [Google Scholar] [CrossRef]
- Predoi, D.; Barsan, M.; Andronescu, E.; Vatasescu-Baltan, R.A.; Costache, M. Hydroxyapatite-iron oxide bioceramic prepared using nano-size powders. J. Optoelectron. A. Mat. 2007, 9, 3609–3613. [Google Scholar]
- Fowler, B.O. Infrared studies of apatites. I. Vibrational assignments for calcium, strontium, and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Degli Esposti, L.; Adamiano, A.; Tampieri, A.; Ramire-Rodriguez, G.B.; Siliqi, D.; Giannini, C.; Ivachenko, P.; Martra, G.; Lin, F.H.; Delgado-Lopez, J.M.; et al. Combined Effect of Citrate and Fluoride Ions on Hydroxyapatite Nanoparticles. Cryst. Growth Des. 2020, 20, 3163–3172. [Google Scholar] [CrossRef]
- Eduok, S.; Coulon, F. Engineered nanoparticles in the environments: Interactions with microbial systems and microbial activity. In Microbial Ecotoxicology; Cravo-Laureau, C., Ed.; Springer: Cham, Switzerland, 2017; pp. 63–107. [Google Scholar]
- Valgas, C.; Machado de Souza, S.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microb. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Senesi, G.S.; Manzini, D.; De Pascale, O. Application of a laser-induced breakdown spectroscopy handheld instrument to the diagnostic analysis of stone monuments. Appl. Geochem. 2018, 96, 87–91. [Google Scholar] [CrossRef]




| HAp | Assignment |
|---|---|
| 3571 | νs(OH) |
| 1637 | δ(H2O) |
| 1089 | |
| 1046 | ν3(PO4)3− |
| 962 | ν1(PO4)3− |
| 632 | δ(OH) |
| 601 | |
| 570 | ν4(PO4)3− |
| 473 | ν2(PO4)3− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capitelli, F.; Dida, B.; Ventura, G.D.; Baldassarre, F.; Capelli, D.; Senesi, G.S.; Mele, A.; Siliqi, D. Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage. Proceedings 2020, 62, 11. https://doi.org/10.3390/proceedings2020062011
Capitelli F, Dida B, Ventura GD, Baldassarre F, Capelli D, Senesi GS, Mele A, Siliqi D. Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage. Proceedings. 2020; 62(1):11. https://doi.org/10.3390/proceedings2020062011
Chicago/Turabian StyleCapitelli, Francesco, Bujar Dida, Giancarlo Della Ventura, Francesco Baldassarre, Davide Capelli, Giorgio S. Senesi, Altin Mele, and Dritan Siliqi. 2020. "Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage" Proceedings 62, no. 1: 11. https://doi.org/10.3390/proceedings2020062011
APA StyleCapitelli, F., Dida, B., Ventura, G. D., Baldassarre, F., Capelli, D., Senesi, G. S., Mele, A., & Siliqi, D. (2020). Functional Nano-Hydroxyapatite for Applications in Conservation of Stony Monuments of Cultural Heritage. Proceedings, 62(1), 11. https://doi.org/10.3390/proceedings2020062011









