Characterization of the Plant-Associated Bacterial Microbiota of the Mexican Medicinal Species Bouvardia ternifolia †
Abstract
:1. Introduction
2. Material and Methods
2.1. Sampling
2.2. DNA Extraction
2.3. DNA Massive Sequencing
2.4. Microbial Abundance and Diversity Analyses
2.5. Determination of Shared OTUs, Differential Abundance and Core Endophyte Bacterial Taxa
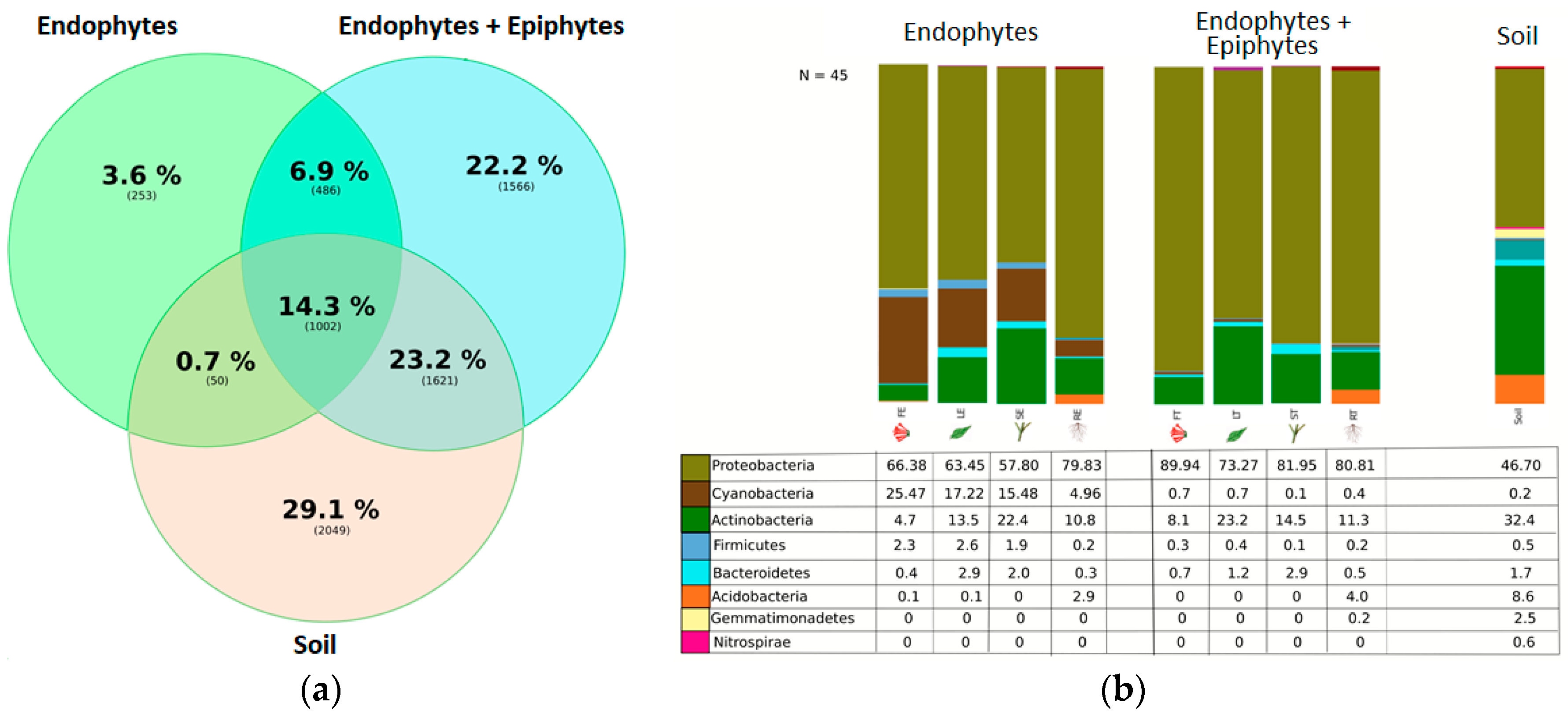
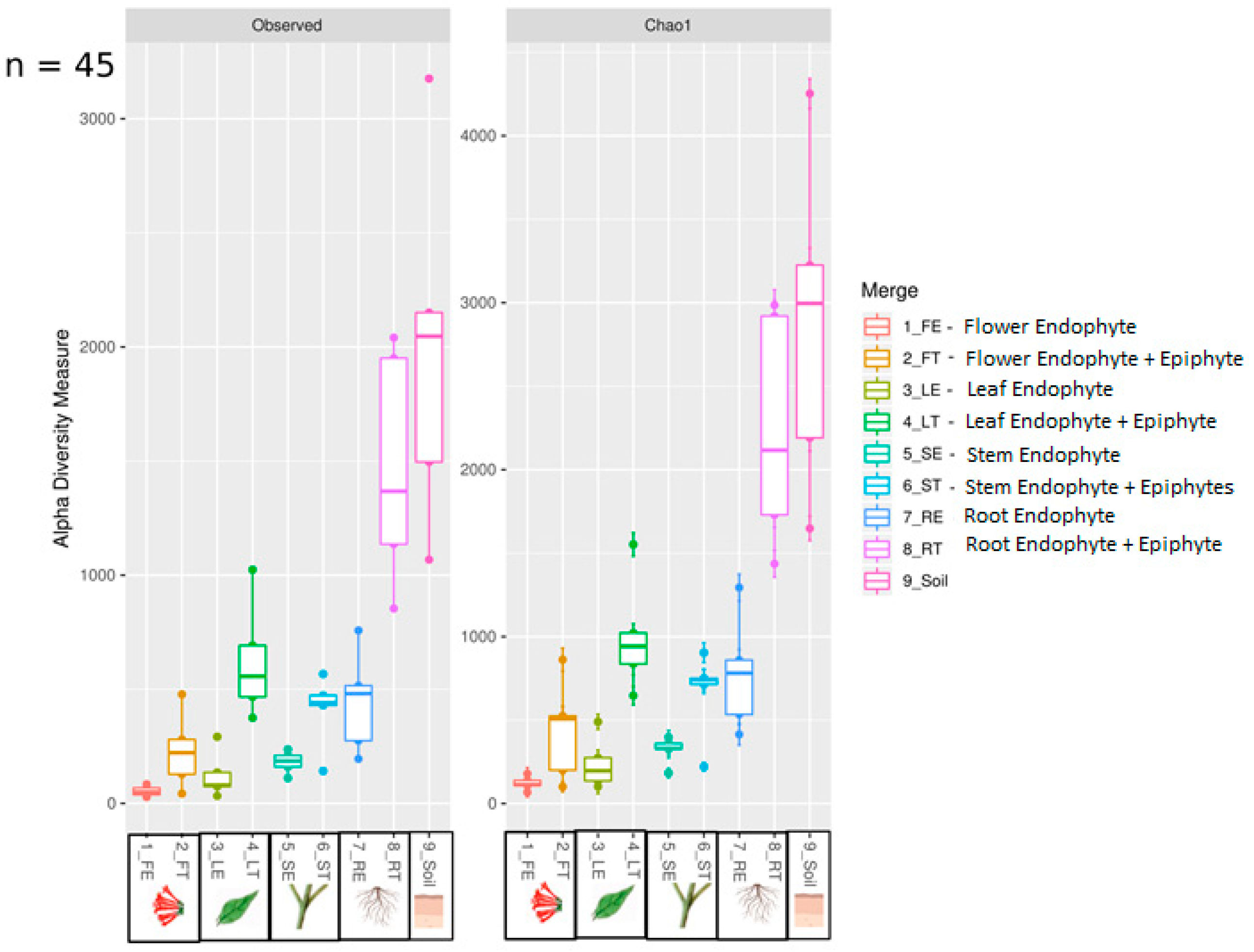
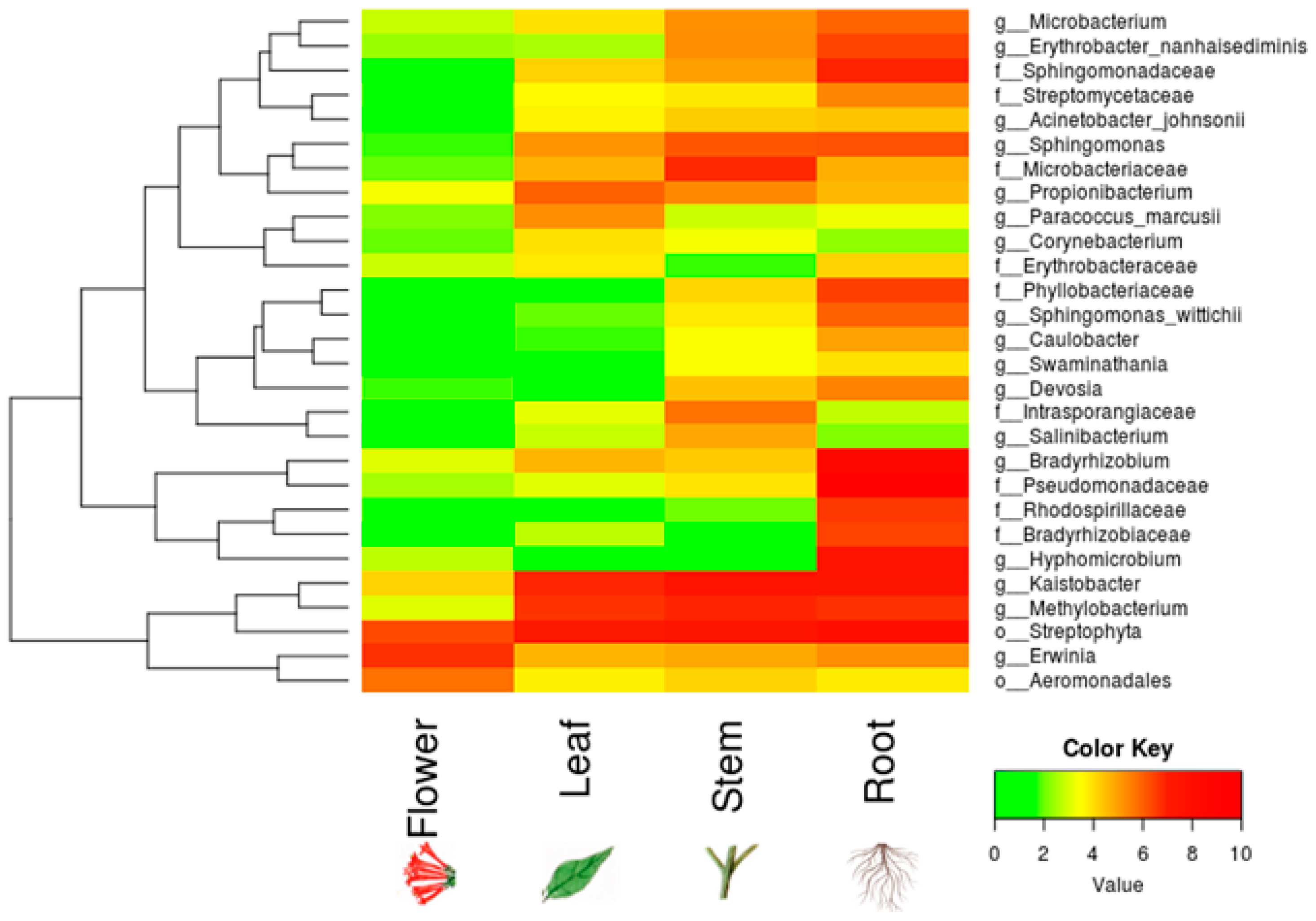
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Ferrer, J.E.; Pérez-Terán, Y.Y.; Román-Ramos, R.; Tortoriello, J. Antitoxin activity of plants used in Mexican traditional medicine against scorpion poisoning. Phytomedicine 2005, 12, 116–122. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, G.; Huerta-Reyes, M.; González-Cortazar, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Silva-García, R.; Román-Ramos, R.; Aguilar-Rojas, A. Anti-inflammatory, antioxidant and anti-acetylcholinesterase activities of Bouvardia ternifolia: Potential implications in Alzheimer’s disease. Arch. Pharmacal Res. 2015, 38, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Jolad, S.D.; Hoffman, J.J.; Torrance, S.J.; Wiedhopf, R.M.; Cole, J.R.; Arora, S.K.; Bates, R.B.; Gargiulo, R.L.; Kriek, G.R. Bouvardin and Deoxybouvardin, Antitumor Cyclic Hexapeptides from Bouvardia ternifolia (Rubiaceae). J. Am. Chem. Soc. 1977, 99, 8040–8044. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Hamady, M.; Lauber, C.L.; Knight, R. The influence of sex, handedness, and washing on the diversity of hand surface bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 17994–17999. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 18 January 2019).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- CONABIO. Capital Natural de México, Vol. II: Conocimiento actual de la biodiversidad. Anexo: Catalogo taxonómico de especies; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2009; ISBN 978-607-7607-08-3. [Google Scholar]
- Afzal, I.; Shinwari, Z.K.; Sikandar, S.; Shahzad, S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019, 221, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S.; Jiang, Q.; Bai, Y.; Shen, G.; Li, S.; Ding, W. Using community analysis to explore bacterial indicators for disease suppression of tobacco bacterial wilt. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Alex, T.H.H.; Te Ngoh, S.; Prithiviraj, B.; Ji, L. Leaf-residing Methylobacterium species fix nitrogen and promote biomass and seed production in Jatropha curcas. Biotechnol. Biofuels 2015, 8, 222. [Google Scholar] [CrossRef] [PubMed]
- Campisano, A.; Ometto, L.; Compant, S.; Pancher, M.; Antonielli, L.; Yousaf, S.; Varotto, C.; Anfora, G.; Pertot, I.; Sessitsch, A.; et al. Interkingdom transfer of the acne-causing agent, propionibacterium acnes, from human to grapevine. Mol. Biol. Evol. 2014, 31, 1059–1065. [Google Scholar] [CrossRef]
- Sun, L.N.; Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresour. Technol. 2010, 101, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.G.; Beltran, L.P.; Buerkle, C.A.; Cook, D.; Gardner, D.R.; Parchman, T.L.; Forister, M.L. A suite of rare microbes interacts with a dominant, heritable, fungal endophyte to influence plant trait expression. bioRxiv 2019, 608729. [Google Scholar] [CrossRef] [PubMed]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos-Flores, L.E.; Espinosa-Torres, S.D.; Hernández-Quiroz, F.; Piña-Escobedo, A.; Cruz-Narváez, Y.; Velázquez-Escobar, F.; Süssmuth, R.; García-Mena, J. Characterization of the Plant-Associated Bacterial Microbiota of the Mexican Medicinal Species Bouvardia ternifolia . Proceedings 2020, 66, 34. https://doi.org/10.3390/proceedings2020066034
Villalobos-Flores LE, Espinosa-Torres SD, Hernández-Quiroz F, Piña-Escobedo A, Cruz-Narváez Y, Velázquez-Escobar F, Süssmuth R, García-Mena J. Characterization of the Plant-Associated Bacterial Microbiota of the Mexican Medicinal Species Bouvardia ternifolia . Proceedings. 2020; 66(1):34. https://doi.org/10.3390/proceedings2020066034
Chicago/Turabian StyleVillalobos-Flores, Loan Edel, Samuel David Espinosa-Torres, Fernando Hernández-Quiroz, Alberto Piña-Escobedo, Yair Cruz-Narváez, Francisco Velázquez-Escobar, Roderich Süssmuth, and Jaime García-Mena. 2020. "Characterization of the Plant-Associated Bacterial Microbiota of the Mexican Medicinal Species Bouvardia ternifolia " Proceedings 66, no. 1: 34. https://doi.org/10.3390/proceedings2020066034





