The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources †
Abstract
:1. Introduction
2. Vegetable Oils: A Unique Source of Chemicals and Monomers
3. Branched and Crosslinked Polymers Based on Pristine or Chemically Modified Tryglicerides
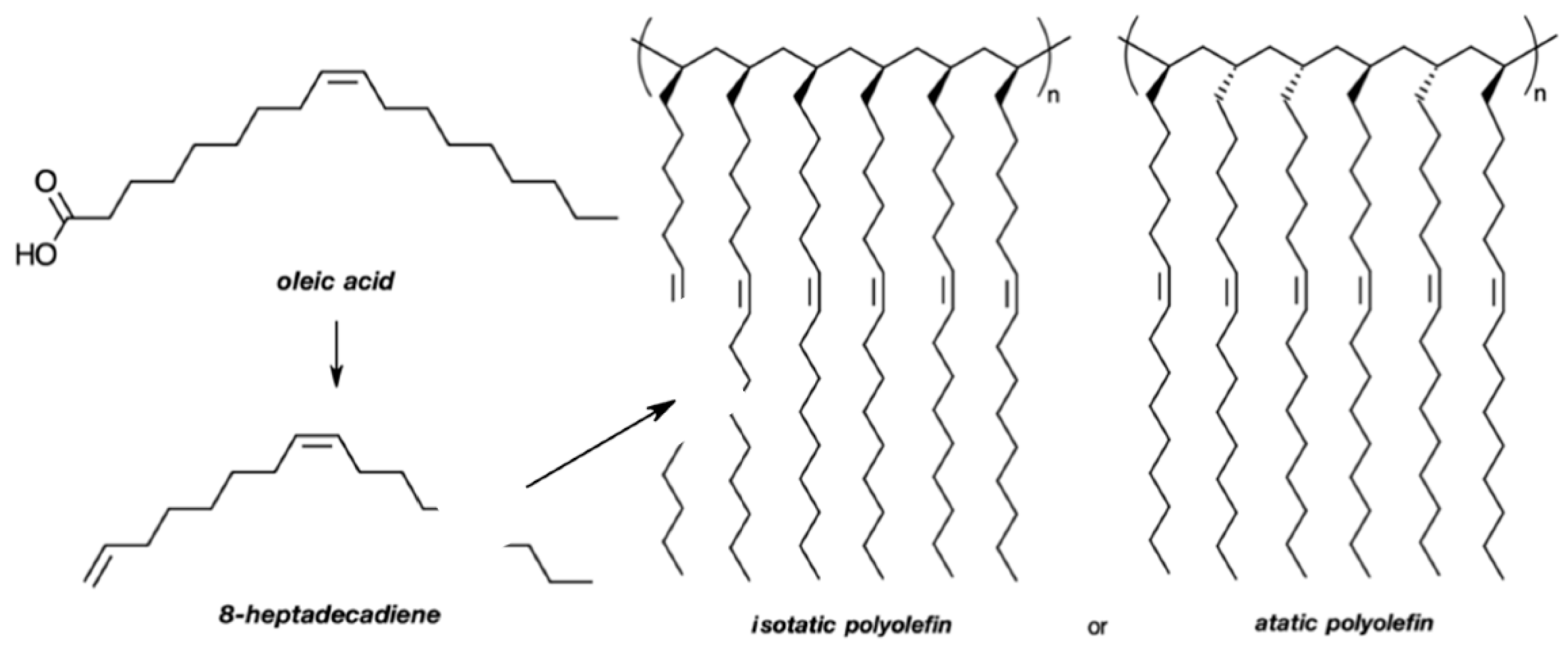
4. Linear Polymers Based on Fatty Acids or Their Ensuing Derivatives
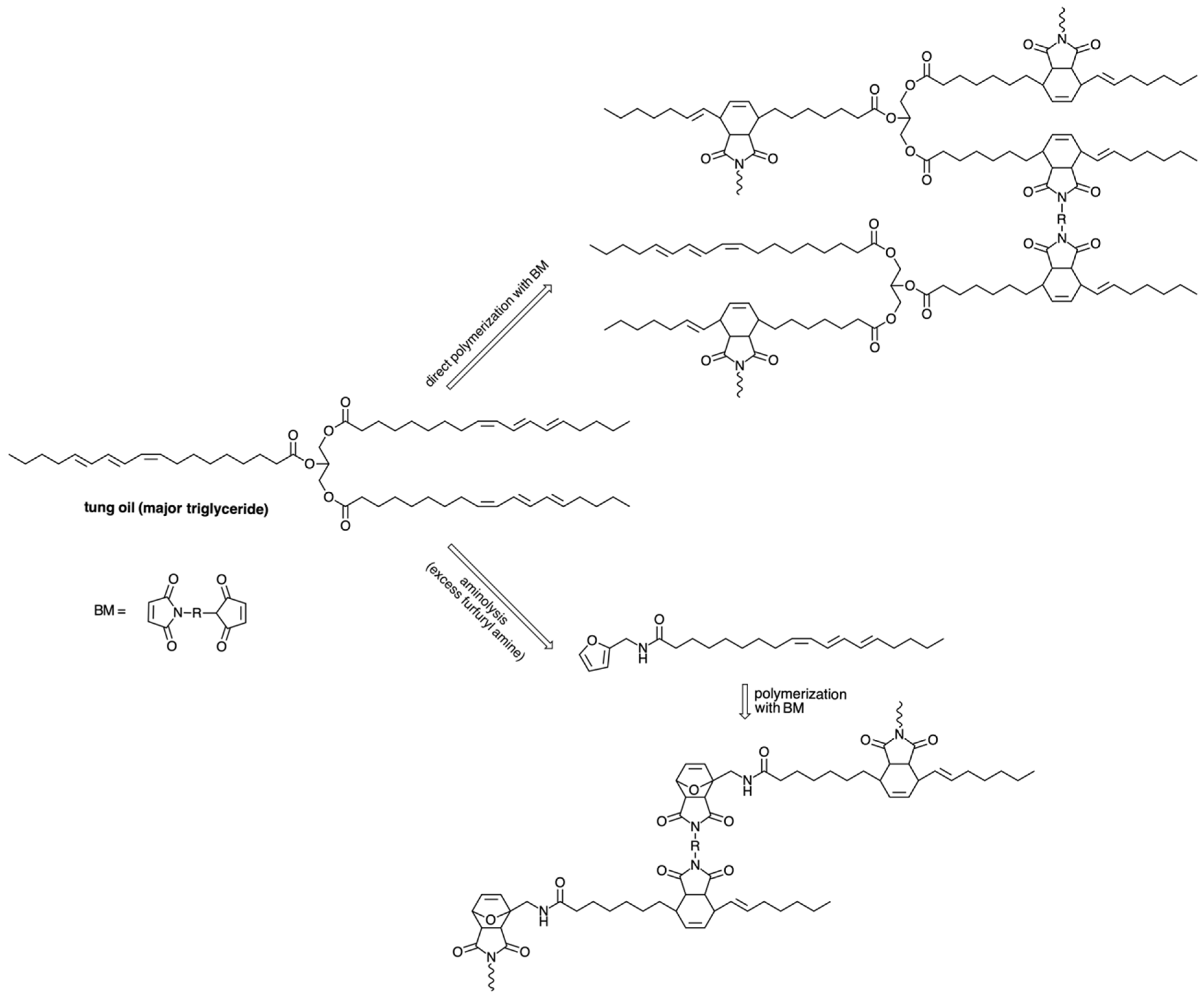
5. The Case of Tung Oil: An Old Ally for Original Materials
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Belgacem, M.N.; Gandini, A. (Eds.) Monomers Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; p. 552. [Google Scholar]
- Gandini, A.; Lacerda, T.M. Polymers from Renewable Resources: Macromolecular Materials for the XXI Century? In Macromolecular Engineering: From Precise Synthesis to Macroscopic Materials and Applications, 2nd ed.; Gnanou, Y., Hadjichristidis, N., Matyjaszewski, K., Muthukumar, M., Sheiko, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; in press. [Google Scholar]
- Kawashima, N.; Yagi, T.; Kojima, K. How do bioplastics and fossil-based plastics play in a circular economy? Macromol. Mater. Eng. 2019, 304, 1900383. [Google Scholar] [CrossRef]
- Hillmyer, M.A. The promise of plastics from plants. Science 2017, 358, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M. Polymers from Plant Oils, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA; Scrivener Publishing: Beverly, MA, USA, 2019. [Google Scholar]
- Demchuk, Z.; Wu, N.; Pourhashem, G.; Voronov, A. Life cycle environmental impact considerations in the design of soybean oil-based acrylic monomers. ACS Sustain. Chem. Eng. 2020, 8, 12870–12876. [Google Scholar] [CrossRef]
- Latif, F.E.A.; Abidin, Z.Z.; Cardona, F.; Biak, D.R.A.; Abdan, K.; Tahir, P.M.; Ern, L.K. Bio-resin production through ethylene unsaturated carbon using vegetable oils. Processes 2020, 8, 48. [Google Scholar] [CrossRef]
- Mucci, V.L.; Hormaiztegui, M.E.V.; Aranguren, M.I. Plant oil-based waterborne polyurethanes: A brief review. J. Renew. Mater. 2020, 8, 579–601. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the synthesis of biobased cyclic carbonate to polyhydroxyurethanes: A promising route towards renewable non-isocyanate polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C.K.S. Natural monomers: A mine for functional and sustainable materials—Occurrence, chemical modification and polymerization. Progr. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Molina-Gutierrez, S.; Ladmiral, V.; Bongiovanni, R.; Caillol, S.; Lacroix-Desmazes, P. Radical polymerization of biobased monomers in aqueous dispersed media. Green Chem. 2019, 21, 36–53. [Google Scholar] [CrossRef]
- Lomegè, J.; Lapinte, V.; Negrell, C.; Robin, J.-J.; Caillol, S. Fatty acid-based radically polymerizable monomers: From novel poly(meth)acrylates to cutting-edge properties. Biomacromolecules 2019, 20, 4–26. [Google Scholar] [CrossRef]
- Web of Science. Available online: www.webofknowledge.com (accessed on 8 September 2020).
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Available online: https://www.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 24 September 2020).
- Biermann, U.; Friedt, W.; Lang, S.; Lühs, W.; Machmüller, G.; Metzger, J.O.; Klaas, M.R.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 2000, 39, 2206–2224. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Türünç, O.; Billiet, S.; Bruycker, K.; Ouardad, S.; Winne, J.; Du Prez, F.E. From plant oils to plant foils: Straightforward functionalization and crosslinking of natural plant oils with triazolinediones. Eur. Polym. J. 2015, 65, 286–297. [Google Scholar] [CrossRef]
- Llevot, A. Sustainable synthetic approaches for the preparation of plant oil-based thermosets. J. Am. Oil Chem. Soc. 2017, 94, 169–186. [Google Scholar] [CrossRef]
- Abbasi, A.; Nasef, M.M.; Yahya, W.Z.N. Copolymerization of vegetable oils and bio-based monomers with elemental sulfur: A new promising route for bio-based polymers. Sustain. Chem. Pharm. 2019, 13, 100158. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying waste to mercury: Inexpensive sorbents made from sulfur and recycled cooking oils. Chem. Eur. J. 2017, 23, 16219–16230. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-step synthesis of novel renewable vegetable oil-based acrylate prepolymers and their application in UV-curable coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Ecochard, Y.; Auvergne, R.; Boutevin, B.; Caillol, S. Linseed oil-based thermosets by Aza-Michael polymerization. Eur. J. Lipid Sci. Technol. 2020, 122, 1900145. [Google Scholar] [CrossRef]
- Echeverri, D.A.; Pérez, W.A.; Inciarte, H.C.; Rios, L.A. Accelerated weathering behavior of castor oil bio-based thermosets. J. Appl. Polym. Sci. 2020, 137, e49509. [Google Scholar] [CrossRef]
- Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Castor-oil derived nonhalogenated reactive flame-retardant-based polyurethane foams with significant reduced heat release rate. J. Appl. Polym. Sci. 2019, 136, 47276. [Google Scholar] [CrossRef]
- Nakamura, K.; Nishimura, Y. Polyurethane foam derived from waste vegetable oil. Kobunshi Ronbunshu 1993, 50, 881–886. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Polyurethanes from seed oil-based polyols: A review of synthesis, mechanical and thermal properties. Ind. Crops Prod. 2019, 142, 111841. [Google Scholar] [CrossRef]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame retardancy of bio-based polyurethanes: Opportunities and challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Gholami, H.; Yeganeh, H. Vegetable oil-based polyurethanes as antimicrobial wound dressings: In vitro and in vivo evaluation. Biomed. Mater. 2020, 15, 045001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Y.; Wang, Z.; Han, Y.; Tang, C. Plant oil and lignin-derived elastomers via thermal azide−alkyne cycloaddition click chemistry. ACS Sustain. Chem. Eng. 2019, 7, 2593–2601. [Google Scholar] [CrossRef]
- Sajjad, H.; Tolman, W.B.; Reineke, T.M. Block copolymer pressure-sensitive adhesives derived from fatty acids and triacetic acid lactone. ACS Appl. Polym. Mater. 2020, 2, 2719–2728. [Google Scholar] [CrossRef]
- Fleckhaus, A.; Fokou, P.A.; Klaassen, G.; Klaas, M.R. One-pot catalytic copolymerization of unsaturated plant oils or fatty acid methyl esters with ethylene. Eur. J. Lipid Sci. Technol. 2019, 121, 1700429. [Google Scholar] [CrossRef]
- Ohtake, K.; Onose, Y.; Kuwabara, J.; Kanbara, T. Synthesis and characterization of a thermally crosslinkable polyolefin from oleic acid. J. Polym. Sci. A1 2019, 57, 85–89. [Google Scholar] [CrossRef]
- Ohtake, K.; Onose, Y.; Kuwabara, J.; Kanbara, T. Postfunctionalization of reactive polyolefins derived from fatty acids. React. Funct. Polym. 2019, 139, 17–24. [Google Scholar] [CrossRef]
- Mecking, S. Polyethylene-like materials from plant oils. Phil. Trans. R. Soc. A 2020, 378, 20190266. [Google Scholar] [CrossRef]
- Liu, Y.; Mecking, S. A synthetic polyester from plant oil feedstock by functionalizing polymerization. Angew. Chem. Int. Ed. 2019, 58, 3346–3350. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Castro, C.; Gomez, M.D.; Nava, M.G.; García, J.M.R.; Uribe, L.E.L. Biobased polyester obtained from bifunctional monomers through metathesis of fatty acids as precursor to synthesis of polyurethanes. J. Appl. Polym. Sci. 2019, 136, 47095. [Google Scholar] [CrossRef]
- Liu, X.; Jain, T.; Liu, Q.; Joy, A. Structural insight into the viscoelastic behaviour of elastomeric polyesters: Effect of the nature of fatty acid side chains and the degree of unsaturation. Polym. Chem. 2020, 11, 5216–5224. [Google Scholar] [CrossRef]
- Haia, T.A.P.; Neelakantan, N.; Tessman, M.; Sherman, S.; Griffin, G.; Pomeroy, R.S.; Mayfield, S.; Burkart, M. Flexible polyurethanes, renewable fuels, and flavorings from a microalgae oil waste stream. Green Chem. 2020, 22, 3088–3094. [Google Scholar] [CrossRef]
- Park, M.S.; Lee, S.; Kim, AR.; Choi, I.; Shin, J.; Kim, Y.-W. Toughened and hydrophobically modified polyamide 11 copolymers with dimer acids derived from waste vegetable oil. J. Appl. Polym. Sci. 2019, 136, 47174. [Google Scholar] [CrossRef]
- Durand, P.-L.; Bregè, A.; Chollet, G.; Grau, E.; Cramail, H. Simple and efficient approach toward photosensitive biobased aliphatic polycarbonate materials. ACS Macro Lett. 2018, 7, 250–254. [Google Scholar] [CrossRef]
- Cui, S.; Borgemenke, J.; Liu, Z.; Li, Y. Recent advances of “soft” bio-polycarbonate plastics from carbon dioxide and renewable bio-feedstocks via straightforward and innovative routes. J. CO2 Util. 2019, 34, 40–52. [Google Scholar] [CrossRef]
- Panchal, B.; Chang, T.; Qin, S.; Sun, Y.; Wang, J.; Bian, K. Optimization and kinetics of tung nut oil transesterification with methanol using novel solid acidic ionic liquid polymer as catalyst for methyl ester synthesis. Renew. Energy 2020, 151, 796–804. [Google Scholar] [CrossRef]
- He, Z.; Qiana, J.; Qua, L.; Yan, N.; Yi, S. Effects of Tung oil treatment on wood hygroscopicity, dimensional stability and thermostability. Ind. Crops Prod. 2019, 140, 111647. [Google Scholar] [CrossRef]
- Li, F.; Larock, R.C. Thermosetting polymers from cationic copolymerization of tung oil: Synthesis and characterization. J. Appl. Polym. Sci. 2000, 78, 1044–1056. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile synthesis and characterization of novel multi-functional bio-based acrylate prepolymers derived from tung oil and its application in UV-curable coatings. Ind. Crops Prod. 2019, 138, 111585. [Google Scholar] [CrossRef]
- Man, L.; Hu, Y.; Feng, Y.; Zhang, C.; Yuan, T.; Yang, Z. Facile synthesis of a novel bio-based methacrylate monomer derived from tung oil and its application for solvent-free thermosetting coatings. Ind. Crops Prod. 2019, 133, 348–356. [Google Scholar] [CrossRef]
- Sain, S.; Åkesson, D.; Skrifvars, M. Synthesis and properties of thermosets from tung oil and furfuryl methacrylate. Polymers 2020, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, T.M.; Gandini, A. The cationic polymerization of tung oil and its fatty-acid methyl ester. Ind. Crops Prod. 2020, 157, 112886. [Google Scholar] [CrossRef]
- Ribeiro, B.O.; Valério, V.S.; Gandini, A.; Lacerda, T.M. Copolymers of xylan-derived furfuryl alcohol and natural oligomeric tung oil derivatives. Int. J. Biol. Macromol. 2020, 164, 2497–2511. [Google Scholar] [CrossRef]
- Lacerda, T.M.; Carvalho, A.J.F.; Gandini, A. Two alternative approaches to the Diels-Alder polymerization of tung oil. RSC Adv. 2014, 4, 26829–26837. [Google Scholar] [CrossRef]
- Li, M.; Ding, H.; Yang, X.; Xu, L.; Xia, J.; Li, S. Preparation and properties of self-healing polyurethane elastomer derived from tung-oil-based polyphenol. ACS Omega 2020, 5, 529–536. [Google Scholar] [CrossRef]
- Feng, Y.; Man, L.; Hu, Y.; Chen, L.; Xie, B.; Zhang, C.; Yuan, T.; Yang, Z. One-pot synthesis of polyurethane-imides with tailored performance from castor and tung oil. Progr. Org. Coat. 2019, 132, 62–69. [Google Scholar] [CrossRef]
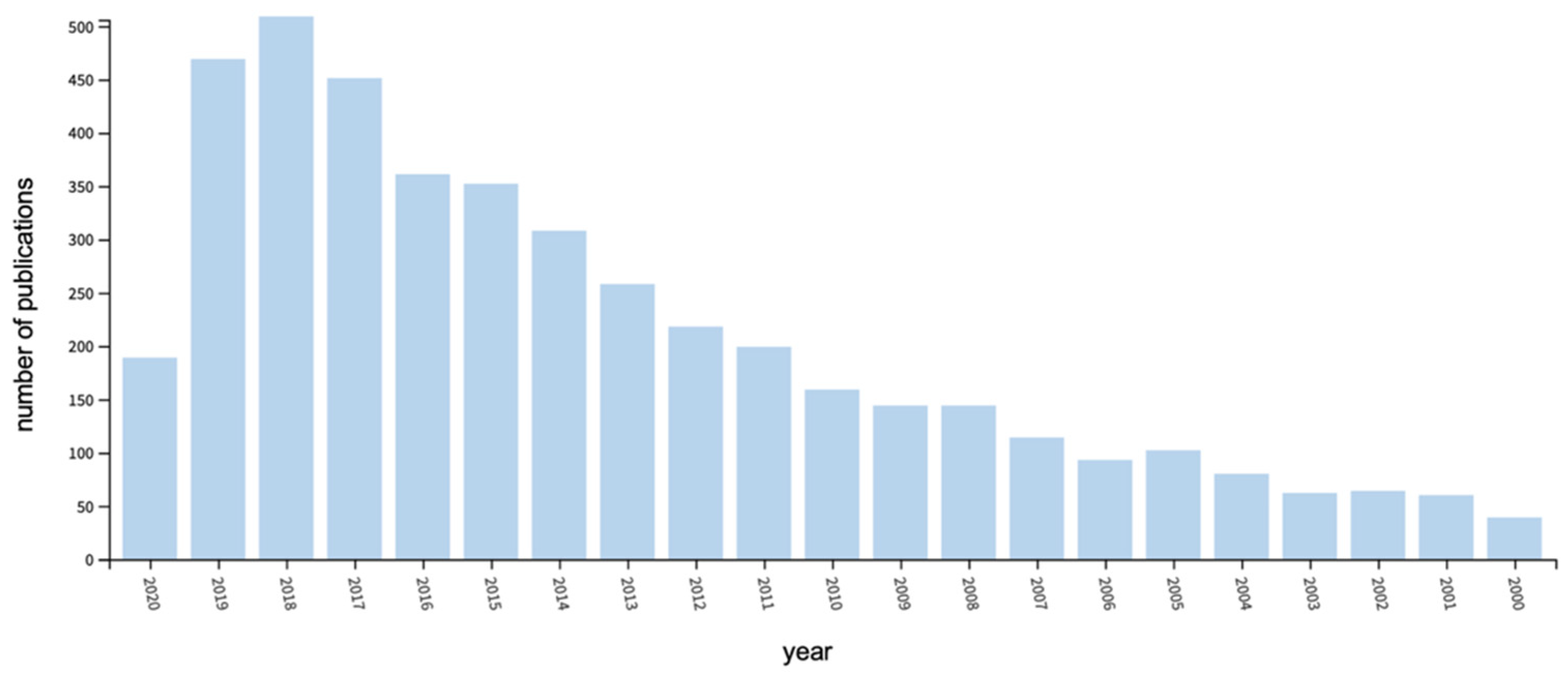
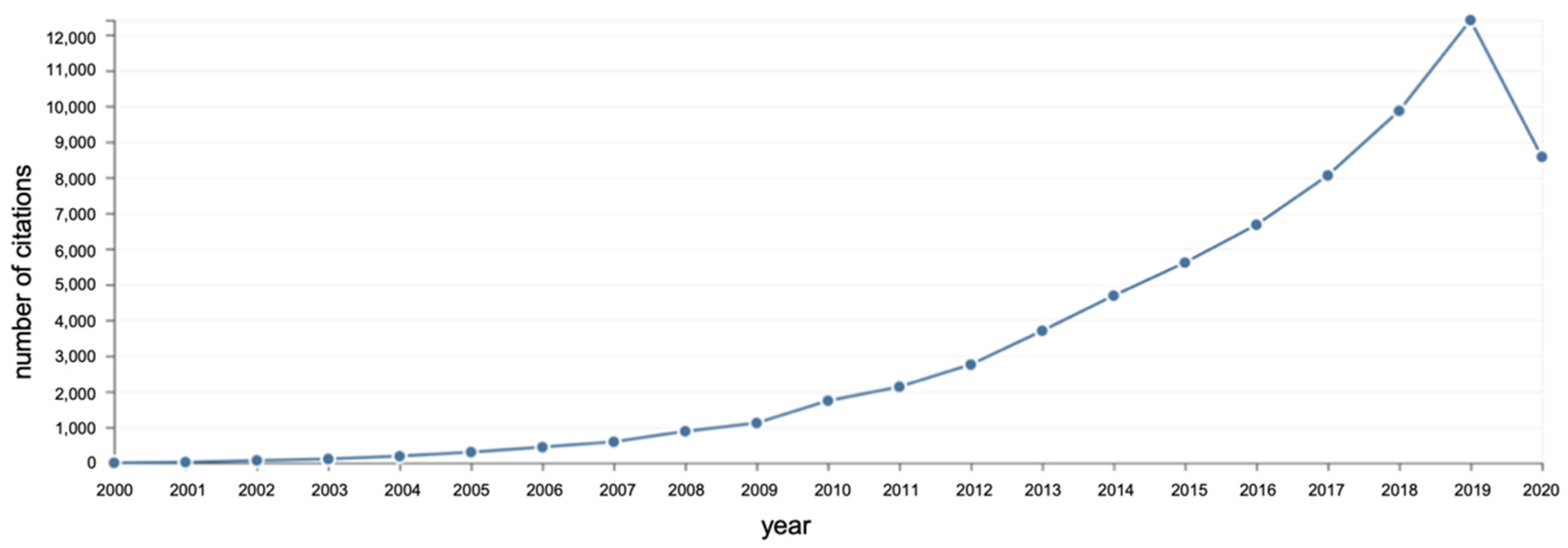
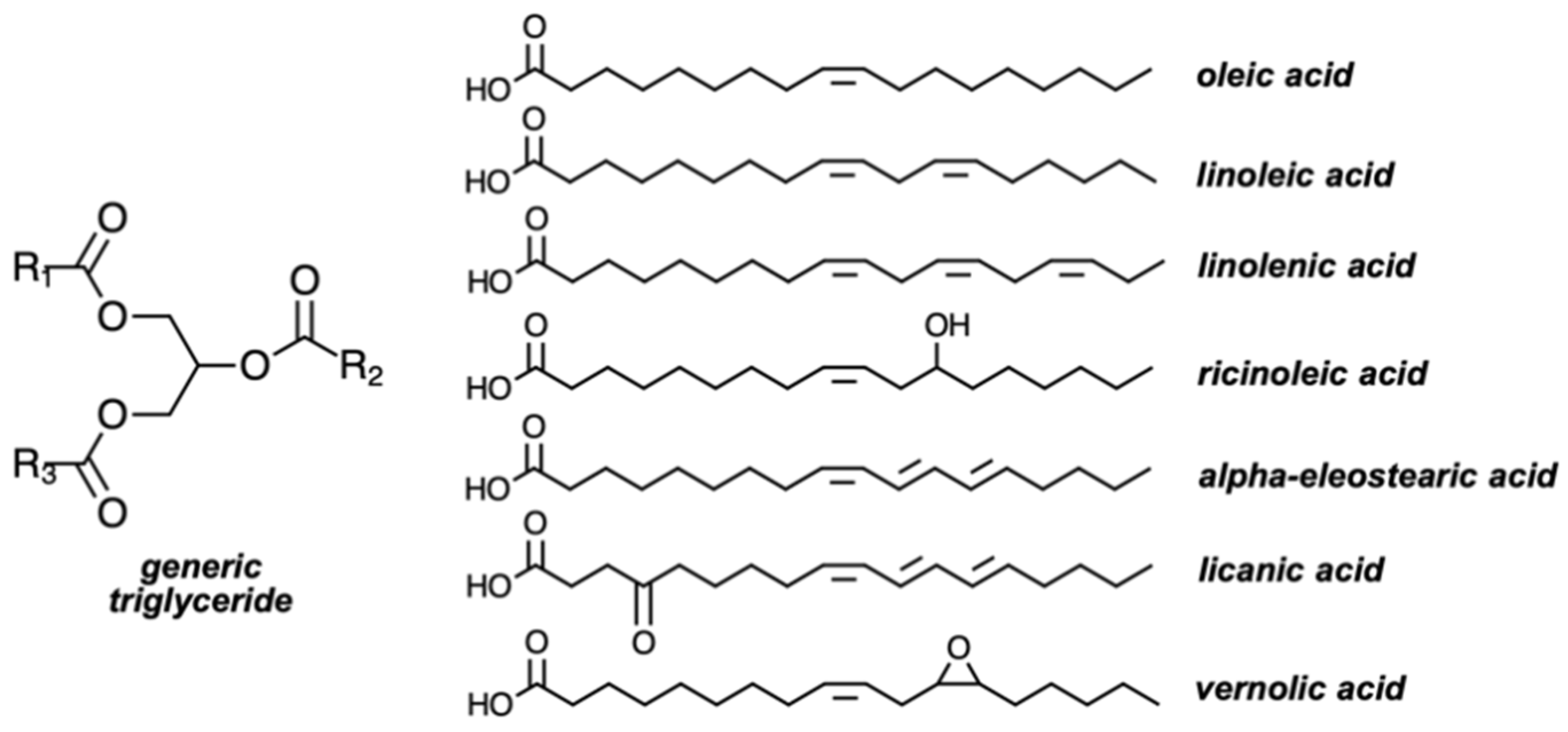
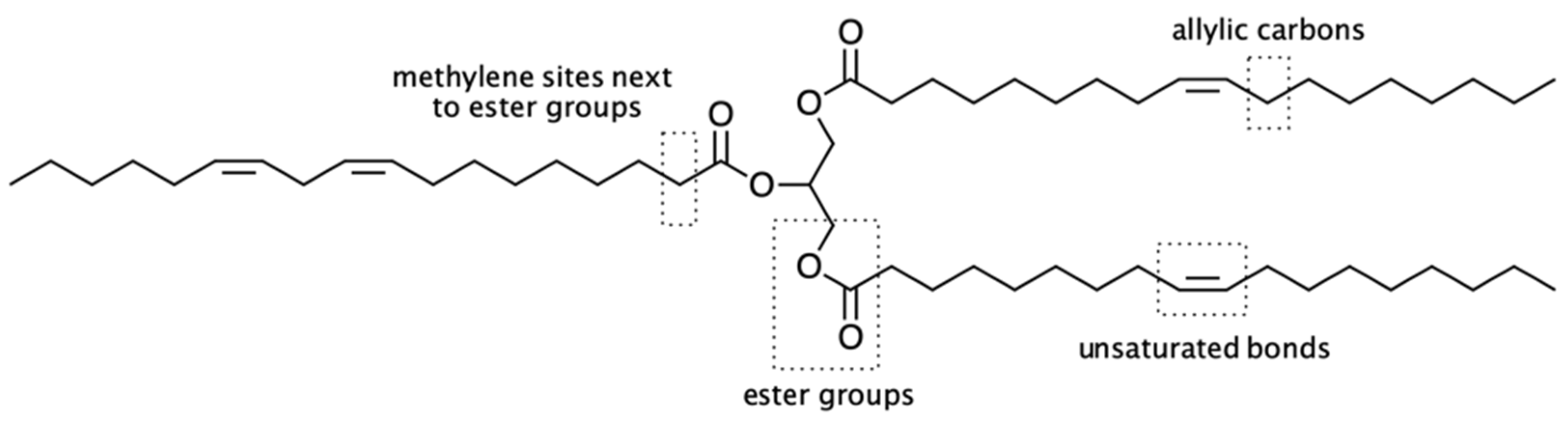


| Fatty Acid (Number of Carbon Atoms) | Iodine Value | |
|---|---|---|
| Acid | Triglyceride | |
| Palmitoleic (C16) | 99.8 | 95 |
| Oleic (C18) | 89.9 | 86 |
| Linoleic (C18) | 181 | 173.2 |
| Linolenic (C18) and α-eleostearic (C18) | 273.5 | 261.6 |
| Ricinoleic (C18) | 85.1 | 81.6 |
| Licanic (C18) | 261 | 258.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandini, A.; Lacerda, T.M. The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Proceedings 2021, 69, 26. https://doi.org/10.3390/CGPM2020-07202
Gandini A, Lacerda TM. The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Proceedings. 2021; 69(1):26. https://doi.org/10.3390/CGPM2020-07202
Chicago/Turabian StyleGandini, Alessandro, and Talita Martins Lacerda. 2021. "The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources" Proceedings 69, no. 1: 26. https://doi.org/10.3390/CGPM2020-07202
APA StyleGandini, A., & Lacerda, T. M. (2021). The Prospering of Macromolecular Materials Based on Plant Oils within the Blooming Field of Polymers from Renewable Resources. Proceedings, 69(1), 26. https://doi.org/10.3390/CGPM2020-07202





