Probiotic Lactobacillus Reuteri Growth Improved under Fucoidan Exposure †
Abstract
:1. Introduction
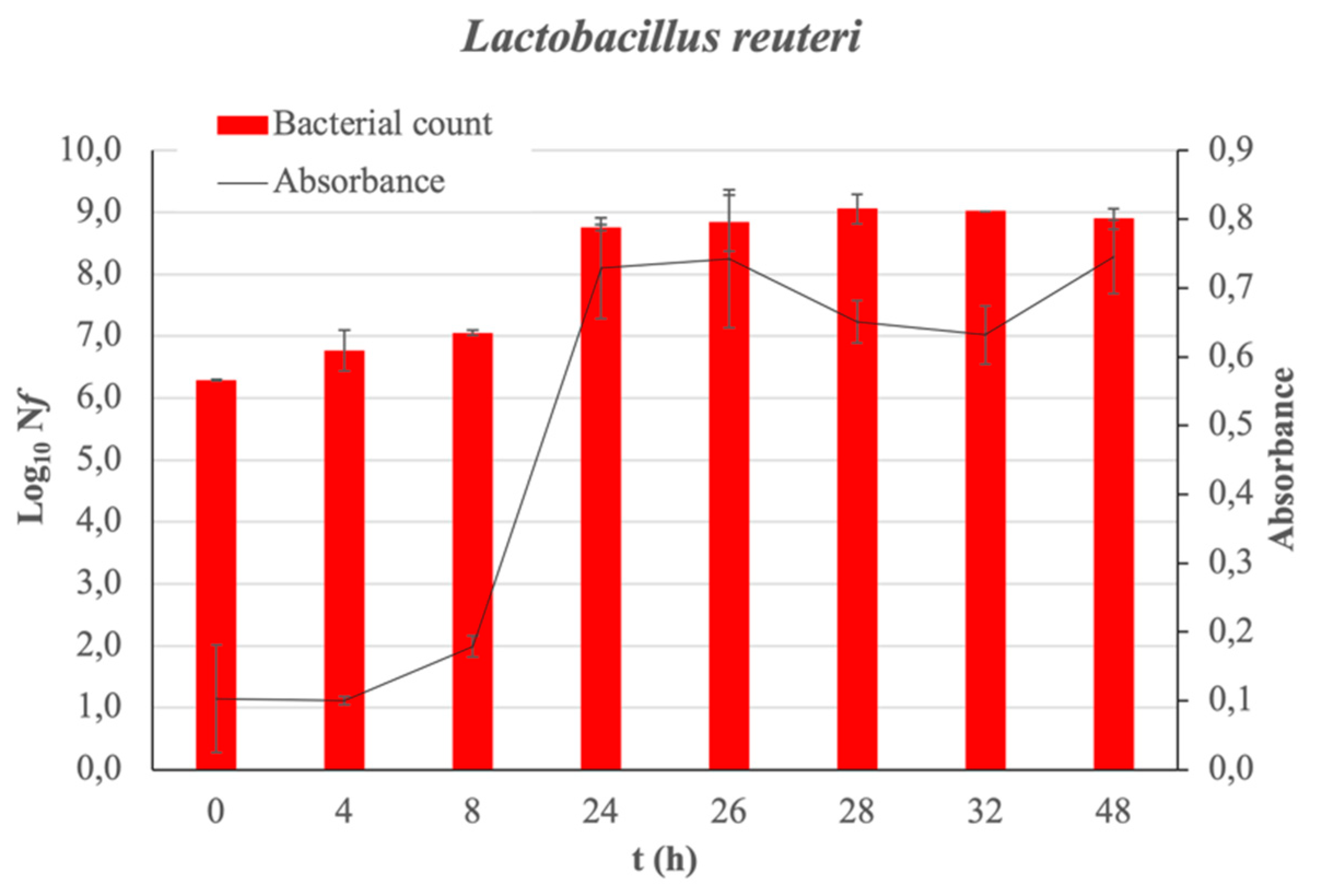
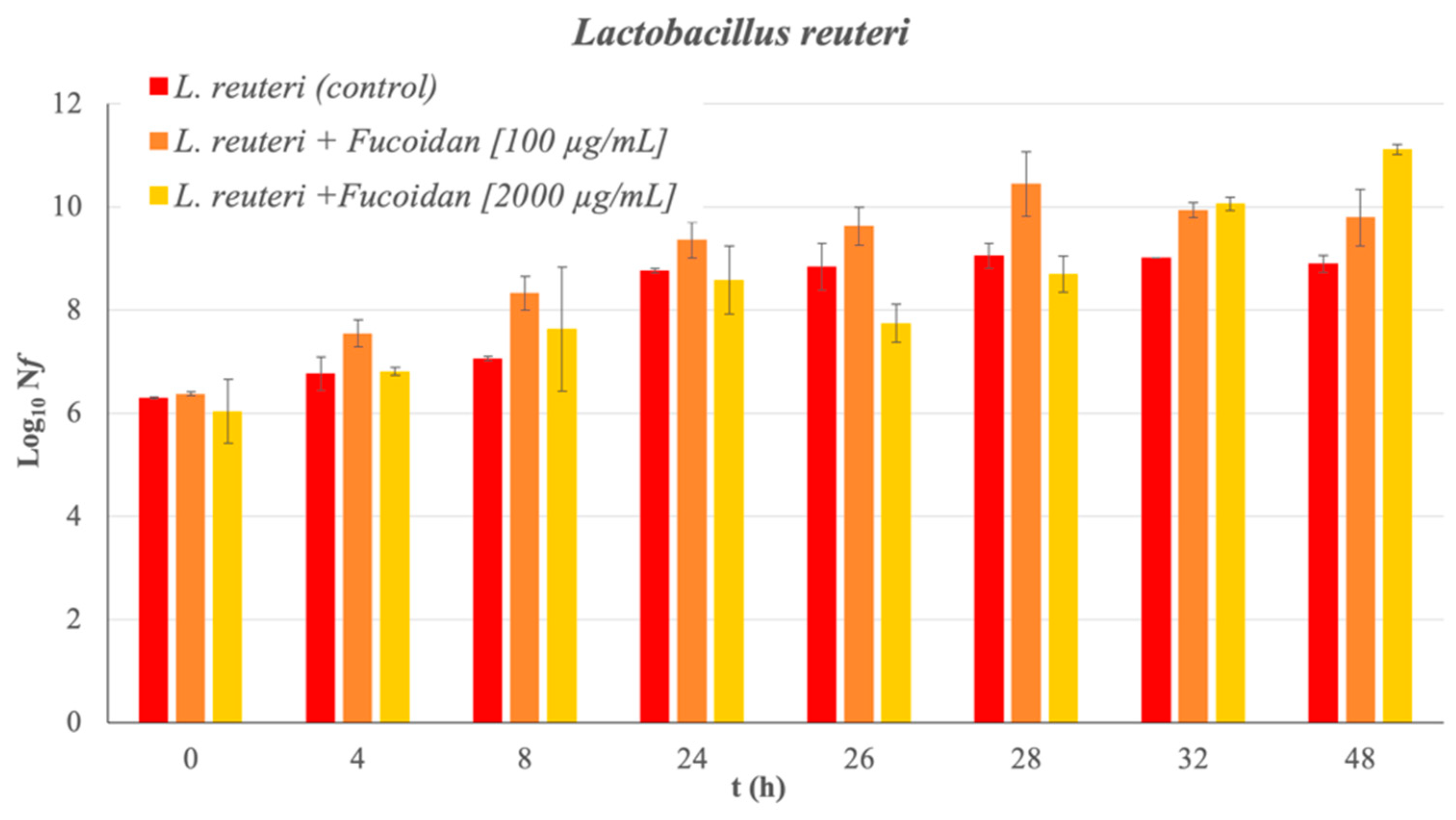
2. Results
3. Discussion
4. Materials and Methods
4.1. Culture Media and other Reagents
4.2. Bacterial Strains
4.3. Fucoidans
4.4. Turbidimetry, Growth Curves and Plate Count
4.5. Mathematical and Statistical Analysis
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GM | Gut microbiota |
| PS | Polysacchacarides |
| OS | Oligosaccharides |
| SCFAs | Short chain fatty acids |
| MRSB | Man Rogose Sharp Broth |
| MRSA | Man Rogose Sharp agar |
| CFU | Colony forming units |
References
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Tinahones, F.J. La importancia de la microbiota en la obesidad. Rev. Esp. Endocrinol. Pediatr. 2017, 8, 16–20. [Google Scholar]
- Icaza-Chávez, M. Microbiota intestinal en la salud y la enfermedad. Rev. Gastroenterol. México 2013, 78, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef]
- Zhuang, L.; Chen, H.; Zhang, S.; Zhuang, J.; Li, Q.; Feng, Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genom. Proteom. Bioinform. 2019, 17, 13–25. [Google Scholar] [CrossRef]
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, Y.k.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri attenuated allergic inflammation induced by HDM in the mouse and modulated gut microbes. PLoS ONE 2020, 15, e0231865. [Google Scholar] [CrossRef]
- Zaporozhets, T.S.; Besednova, N.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Makarenkova, I.D.; Kryzhanovsky, S.P.; Melnikov, V.G. The prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 2014, 40, 1–9. [Google Scholar] [CrossRef]
- Hu, B.; Gong, Q.; Wang, Y.; Ma, Y.; Li, J.; Yu, W. Prebiotic effects of neoagaro-oligosaccharides prepared by enzymatic hydrolysis of agarose. Anaerobe 2006, 12, 260–266. [Google Scholar] [CrossRef]
- Han, Z.L.; Yang, M.; Fu, X.D.; Chen, M.; Su, Q.; Zhao, Y.H.; Mou, H.J. Evaluation of Prebiotic Potential of Three Marine Algae Oligosaccharides from Enzymatic Hydrolysis. Mar. Drugs 2019, 17, 173. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed]
- Murphya, P.; Bello, F.D.; O’Doherty, J.; Arendt, E.K.; Sweeney, T.; Coffey, A. The effects of liquid versus spray-dried Laminaria digitata extract on selected bacterial groups in the piglet gastrointestinal tract (GIT) microbiota. Anaerobe 2013, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- O’Doherty, J.; Dillon, S.; Figat, S.; Sweeney, T. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim. Feed Sci. Technol. 2010, 157, 173–180. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Tuohy, K.; Probert, H.; Smejkal, C.; Gibson, G. Using probiotics and prebiotics to improve gut health. Drug Discov. Today 2003, 8, 692–700. [Google Scholar] [CrossRef]
- Rosenfeldt, V.; Benfeldt, E.; Nielsen, S.D.; Michaelsen, K.F.; Jeppesen, D.L.; Valerius, N.H.; Paerregaard, A. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J. Allergy Clin. Immunol. 2003, 111, 389–395. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Hu, B.; Li, J.; Yu, W. In vivo prebiotic properties of alginate oligosaccharides prepared through enzymatic hydrolysis of alginate. Nutr. Res. 2006, 26, 597–603. [Google Scholar] [CrossRef]
- Palacios-Gorba, C.; Pina, R.; Tortajada, M.; Jiménez-Belenguer, A.; Siguemoto, E.; Rodrigo, D.; Pina-Pérez, M. Caenorhabditis elegans as in vivo model to assess the FUCOIDAN bioactivity preventing Helicobacter pylori infection. Food Funct. 2020, 11, 4525–4534. [Google Scholar] [CrossRef]
- Tanino, Y.; Hashimoto, T.; Ojima, T.; Mizuno, M. F-fucoidan from Saccharina japonica is a novel inducer of galectin-9 and exhibits anti-allergic activity. J. Clin. Biochem. Nutr. 2016, 59, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Niki, T.; Tsutsui, S.; Hirose, S.; Aradono, S.; Sugimoto, Y.; Takeshita, K.; Nishi, N.; Hirashima, M. Galectin-9 Is a High Affinity IgE-binding Lectin with Anti-allergic Effect by Blocking IgE-Antigen Complex Formation. J. Biol. Chem. 2009, 284, 32344–32352. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricós-Muñoz, N.; Maicas, S.; Pina-Pérez, M.C. Probiotic Lactobacillus Reuteri Growth Improved under Fucoidan Exposure. Proceedings 2021, 70, 106. https://doi.org/10.3390/foods_2020-07724
Ricós-Muñoz N, Maicas S, Pina-Pérez MC. Probiotic Lactobacillus Reuteri Growth Improved under Fucoidan Exposure. Proceedings. 2021; 70(1):106. https://doi.org/10.3390/foods_2020-07724
Chicago/Turabian StyleRicós-Muñoz, Neus, Sergi Maicas, and María Consuelo Pina-Pérez. 2021. "Probiotic Lactobacillus Reuteri Growth Improved under Fucoidan Exposure" Proceedings 70, no. 1: 106. https://doi.org/10.3390/foods_2020-07724
APA StyleRicós-Muñoz, N., Maicas, S., & Pina-Pérez, M. C. (2021). Probiotic Lactobacillus Reuteri Growth Improved under Fucoidan Exposure. Proceedings, 70(1), 106. https://doi.org/10.3390/foods_2020-07724





