UHT Treatment on the Stability of Faba Bean Protein Emulsion †
Abstract
:1. Introduction
2. Materials and Methods
Materials
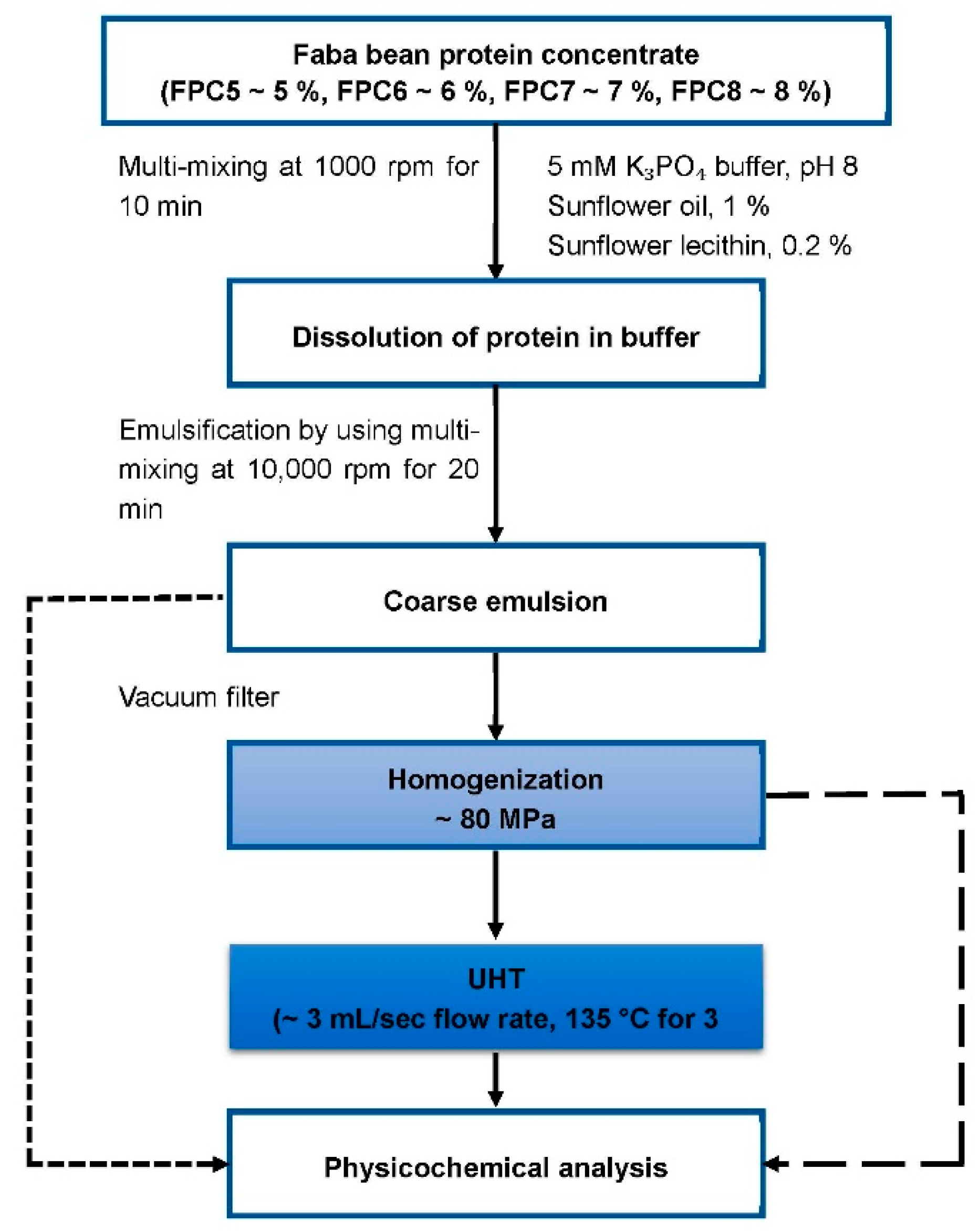
3. Preparation of Emulsion
3.1. Mixing
3.2. Homogenisation of the Emulsion
3.3. Ultra-High Temperature Processing of the Emulsion
4. Physicochemical Properties of the Emulsion
4.1. Particle size Distribution
4.2. Flocculation Index (FI) and Coalescence Index (CI)
4.3. Confocal Laser Scanning Microscopy
4.4. ζ-. Potential
4.5. Surface Hydrophobicity Index (Sₒ)
5. Creaming Index
5.1. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
5.2. Headspace Gas Chromatography-Mass Spectrometry (GC-MS)
5.3. Statistical Analysis
6. Results and Discussion
UHT Processing of Faba Bean Protein Emulsions
7. Physicochemical Properties of the Emulsions
7.1. Particle Size Distribution
7.2. Flocculation Index (FI) and Coalescence Index (CI)
7.3. ζ-Potential
7.4. Surface Hydrophobicity (Sₒ)
7.5. Creaming Index
7.6. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
7.7. Headspace Gas Chromatography-Mass Spectrometry (GC-MS)
8. Conclusions
Acknowledgments
References
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Tan, M.; Øiseth, S.; Buckow, R. An Emerging Segment of Functional Legume-Based Beverages: A Review. Food Rev. Int. 2020, 1–39. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Lacroix, I.M.E. 1-Properties of proteins in food systems: An introduction. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring structure and technological properties of plant proteins using high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef]
- Damodaran, S. Protein Stabilization of Emulsions and Foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Fernandez-Avila, C.; Trujillo, A.J. Ultra-High Pressure Homogenization improves oxidative stability and interfacial properties of soy protein isolate-stabilized emulsions. Food Chem. 2016, 209, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chelikani, V.; Serventi, L. Evaluation of chickpea as alternative to soy in plant-based beverages, fresh and fermented. LWT 2018, 97, 570–572. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. Cyta-J. Food 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Qamar, S.; Bhandari, B.; Prakash, S. Effect of different homogenisation methods and UHT processing on the stability of pea protein emulsion. Food Res. Int. 2019, 116, 1374–1385. [Google Scholar] [CrossRef]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of Fava Bean as Future Protein Supply to Partially Replace Meat Intake in the Human Diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of Physicochemical Properties of 7S and 11S Globulins from Pea, Fava Bean, Cowpea, and French Bean with Those of Soybean—French Bean 7S Globulin Exhibits Excellent Properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef] [PubMed]
- Felix, M.; Cermeño, M.; FitzGerald, R.J. Assessment of the microstructural characteristics and the in vitro bioactive properties of sunflower oil-based emulsions stabilized by fava bean (Vicia faba) protein. Food Hydrocoll. 2019, 97, 105220. [Google Scholar] [CrossRef]
- Raikos, V.; Neacsu, M.; Russell, W.; Duthie, G. Comparative study of the functional properties of lupin, green pea, fava bean, hemp, and buckwheat flours as affected by pH. Food Sci. Nutr. 2014, 2, 802–810. [Google Scholar] [CrossRef]
- Liu, C.; Bhattarai, M.; Mikkonen, K.S.; Heinonen, M. Effects of Enzymatic Hydrolysis of Fava Bean Protein Isolate by Alcalase on the Physical and Oxidative Stability of Oil-in-Water Emulsions. J. Agric. Food Chem. 2019, 67, 6625–6632. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Tsoukala, A.; Papalamprou, E.; Makri, E.; Doxastakis, G.; Braudo, E.E. Adsorption at the air–water interface and emulsification properties of grain legume protein derivatives from pea and broad bean. Colloids Surf. B: Biointerfaces 2006, 53, 203–208. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Thermodynamic compatibility and interactions between Speckled Sugar bean protein and xanthan gum for production of multilayer O/W emulsion. J. Food Sci. Technol. 2018, 55, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Microfluidization as Homogenization Technique in Pea Globulin-Based Emulsions. Food Bioprocess Technol. 2019, 12, 877–882. [Google Scholar] [CrossRef]
- Felix, M.; Cermeño, M.; Romero, A.; FitzGerald, R.J. Characterisation of the bioactive properties and microstructure of chickpea protein-based oil in water emulsions. Food Res. Int. 2019, 121, 577–585. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Dai, C.; Wang, Y.; Chen, W.; Ju, X.; Yuan, J.; He, R. Physical stability and microstructure of rapeseed protein isolate/gum Arabic stabilized emulsions at alkaline pH. Food Hydrocoll. 2019, 88, 50–57. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Physicochemical and emulsification properties of flaxseed (Linum usitatissimum) albumin and globulin fractions. Food Chem. 2018, 255, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Chen, Q.; Li, G.; Zhu, Z.; Yi, J.; Li, C.; Chen, X.; Wang, Y. Properties and Stability of Perilla Seed Protein-Stabilized Oil-in-Water Emulsions: Influence of Protein Concentration, pH, NaCl Concentration and Thermal Treatment. Molecules 2018, 23, 1533. [Google Scholar] [CrossRef] [PubMed]
- Torkamani, A.E.; Juliano, P.; Ajlouni, S.; Singh, T.K. Impact of ultrasound treatment on lipid oxidation of Cheddar cheese whey. Ultrason. Sonochemistry 2014, 21, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Kouchaksaraei, Z.; Varidi, M.; Varidi, M.J.; Pourazarang, H. Influence of processing conditions on the physicochemical and sensory properties of sesame milk: A novel nutritional beverage. Lwt-Food Sci. Technol. 2014, 57, 299–305. [Google Scholar] [CrossRef]
- Ralla, T.; Salminen, H.; Braun, K.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Investigations into the Structure-Function Relationship of the Naturally-Derived Surfactant Glycyrrhizin: Emulsion Stability. Food Biophys. 2020, 15, 288–296. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf. B Biointerfaces 2010, 81, 130–140. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Corredig, M. Heat-induced changes in oil-in-water emulsions stabilized with soy protein isolate. Food Hydrocoll. 2009, 23, 2141–2148. [Google Scholar] [CrossRef]
- Pinto, I.; Buss, A. ζ Potential as a Measure of Asphalt Emulsion Stability. Energy Fuels 2020, 34, 2143–2151. [Google Scholar] [CrossRef]
- Lee, J.; Jo, S.H.; Lim, J. Effect of surface modification of CaCO3 nanoparticles by a silane coupling agent methyltrimethoxysilane on the stability of foam and emulsion. J. Ind. Eng. Chem. 2019, 74, 63–70. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Axelos, M.A.V.; Riaublanc, A. Interfacial properties of fractal and spherical whey protein aggregates. Soft Matter 2011, 7, 7643–7654. [Google Scholar] [CrossRef]
- Zhai, J.l.; Day, L.; Aguilar, M.-I.; Wooster, T.J. Protein folding at emulsion oil/water interfaces. Curr. Opin. Colloid Interface Sci. 2013, 18, 257–271. [Google Scholar] [CrossRef]
- Cioci, F.; Lavecchia, R. Molecular thermodynamics of heat-induced protein unfolding in aqueous media. Aiche J. 1997, 43, 525–534. [Google Scholar] [CrossRef]
- Caplan, M.R.; Schwartzfarb, E.M.; Zhang, S.; Kamm, R.D.; Lauffenburger, D.A. Control of self-assembling oligopeptide matrix formation through systematic variation of amino acid sequence. Biomaterials 2002, 23, 219–227. [Google Scholar] [CrossRef]
- Hosseini, A.; Jafari, S.M.; Mirzaei, H.; Asghari, A.; Akhavan, S. Application of image processing to assess emulsion stability and emulsification properties of Arabic gum. Carbohydr. Polym. 2015, 126, 1–8. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, C.; Kong, X.; Hua, Y. Oxidative modification of soy protein by peroxyl radicals. Food Chem. 2009, 116, 295–301. [Google Scholar] [CrossRef]
- Warsame, A.O.; Michael, N.; O’Sullivan, D.M.; Tosi, P. Identification and quantification of major faba bean seed proteins. J. Agric. Food Chem. 2020, 68, 8535–8544. [Google Scholar] [CrossRef]


 ), homogenised (
), homogenised (  ), and UHT (
), and UHT (  ) on the surface hydrophobicity (So).
) on the surface hydrophobicity (So).
 ), homogenised (
), homogenised (  ), and UHT (
), and UHT (  ) on the surface hydrophobicity (So).
) on the surface hydrophobicity (So).



| Treatment | Emulsion Type | PDI (%) | FI | CI | ζ-Potential (mV) | Creaming Index |
|---|---|---|---|---|---|---|
| FPC5 | coarse | 24.38 ± 0.01 c | 88.81 ± 0.35 d | 101.26 ± 5.24 cd | −27.60 ± 3.25 d | 12.45 ± 0.35 b |
| homogenised | 6.88 ± 0.24 e | 4.79 ± 0.19 e | 63.93 ± 0.34 ef | −24.63 ± 1.50 cd | 7.00 ± 0.28 g | |
| UHT | 21.37 ± 0.71 cd | 105.06 ± 3.66 d | 142.34 ± 9.92 b | −23.10 ± 0.96 bc | 9.25 ± 021 de | |
| FPC6 | coarse | 41.52 ± 5.45 b | 160.82 ± 17.80 c | 101.57 ± 2.24 cd | −23.10 ± 2.10 bc | 13.05 ± 0.21 b |
| homogenised | 10.44 ± 0.13 de | 11.10 ± 2.87 e | 47.81 ± 0.49 f | −21.20 ± 0.80 b | 7.20 ± 0.42 g | |
| UHT | 29.03 ± 1.81 c | 194.98 ± 0.15 ab | 175.32 ± 4.81 a | −21.47 ± 1.10 bc | 10.25 ± 0.50 cd | |
| FPC7 | coarse | 22.81 ± 0.33 c | 182.22 ± 12.42 bc | 110.95 ± 5.30 c | −0.02 ± 0.02 a | 14.55 ± 0.21 a |
| homogenised | 9.17 ± 0.41 e | 10.83 ± 3.06 e | 83.41 ± 7.59 de | 0.08 ± 0.03 a | 7.95 ± 0.21 fg | |
| UHT | 22.89 ± 1.45 c | 212.14 ± 3.91 ab | 191.81 ± 11.82 a | −0.12 ± 0.07 a | 10.40 ± 0.28 c | |
| FPC8 | coarse | 20.89 ± 0.18 cd | 199.74 ± 18.27 ab | 109.06 ± 1.30 c | 0.01 ± 0.07 a | 14.95 ± 0.21 a |
| homogenised | 7.84 ± 0.34 e | 16.10 ± 1.27 e | 78.98 ± 0.32 de | 0.01 ± 0.20 a | 8.40 ± 0.14 ef | |
| UHT | 59.49 ± 7.40 a | 226.50 ± 0.26 a | 197.43 ± 7.08 a | 0.24 ± 0.11 a | 15.15 ± 0.21 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, M.A.; Singh, T.K.; Jegasothy, H.; Buckow, R. UHT Treatment on the Stability of Faba Bean Protein Emulsion. Proceedings 2021, 70, 29. https://doi.org/10.3390/foods_2020-07742
Nawaz MA, Singh TK, Jegasothy H, Buckow R. UHT Treatment on the Stability of Faba Bean Protein Emulsion. Proceedings. 2021; 70(1):29. https://doi.org/10.3390/foods_2020-07742
Chicago/Turabian StyleNawaz, Malik Adil, Tanoj Kumar Singh, Hema Jegasothy, and Roman Buckow. 2021. "UHT Treatment on the Stability of Faba Bean Protein Emulsion" Proceedings 70, no. 1: 29. https://doi.org/10.3390/foods_2020-07742
APA StyleNawaz, M. A., Singh, T. K., Jegasothy, H., & Buckow, R. (2021). UHT Treatment on the Stability of Faba Bean Protein Emulsion. Proceedings, 70(1), 29. https://doi.org/10.3390/foods_2020-07742






