Interactions between L. monocytogenes and P. fluorescens in Dual-Species Biofilms under Simulated Dairy Processing Conditions †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Serotype and Pulsotype of L. monocytogenes Strains
3.2. Biofilm Formation on the Polystyrene Surface
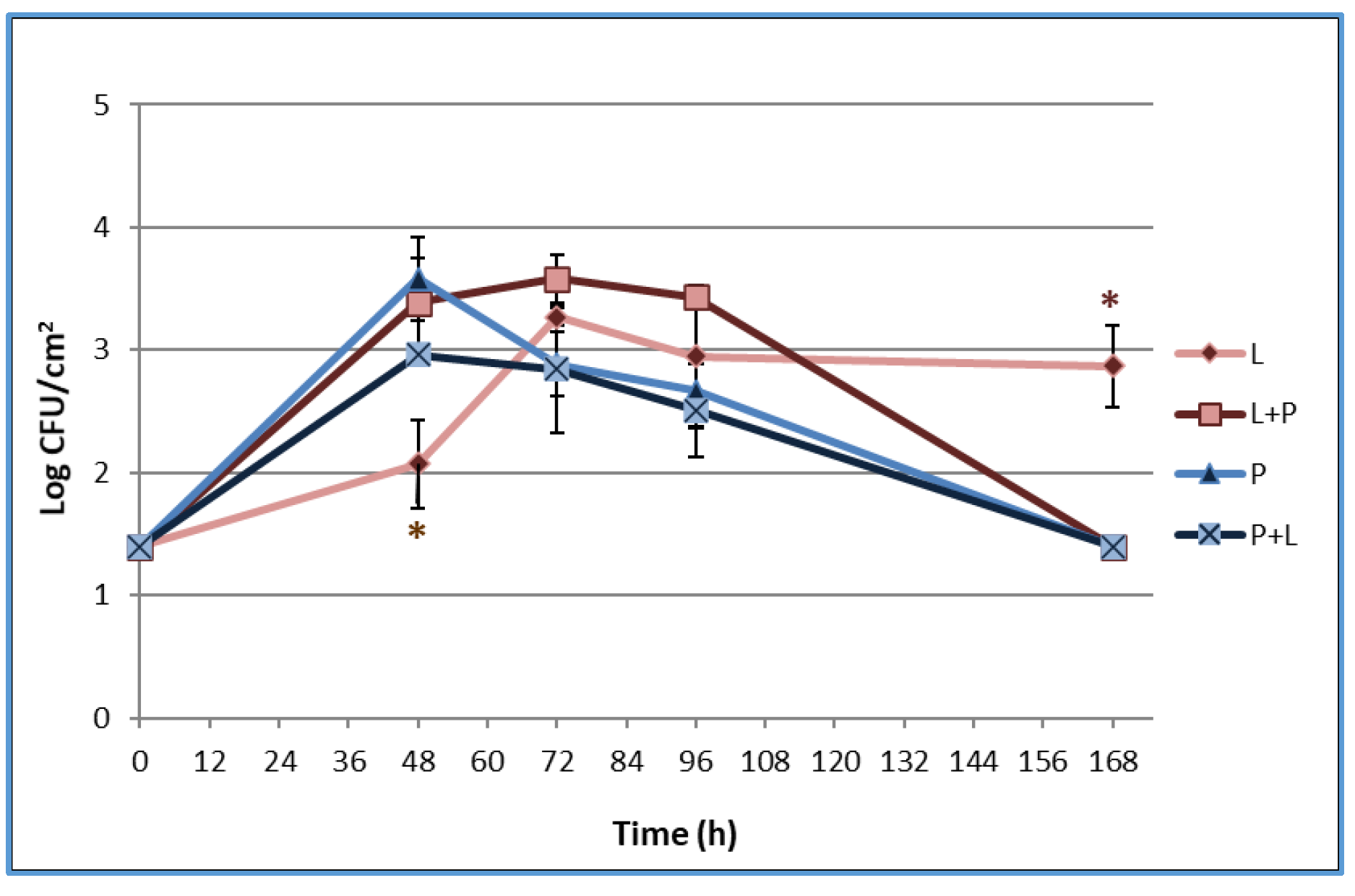
3.3. Biofilm Formation on the Stainless Steel Surface and Enumeration of Planktonic and Sessile Cells
3.4. EPS Analysis by Carbohydrates Quantification
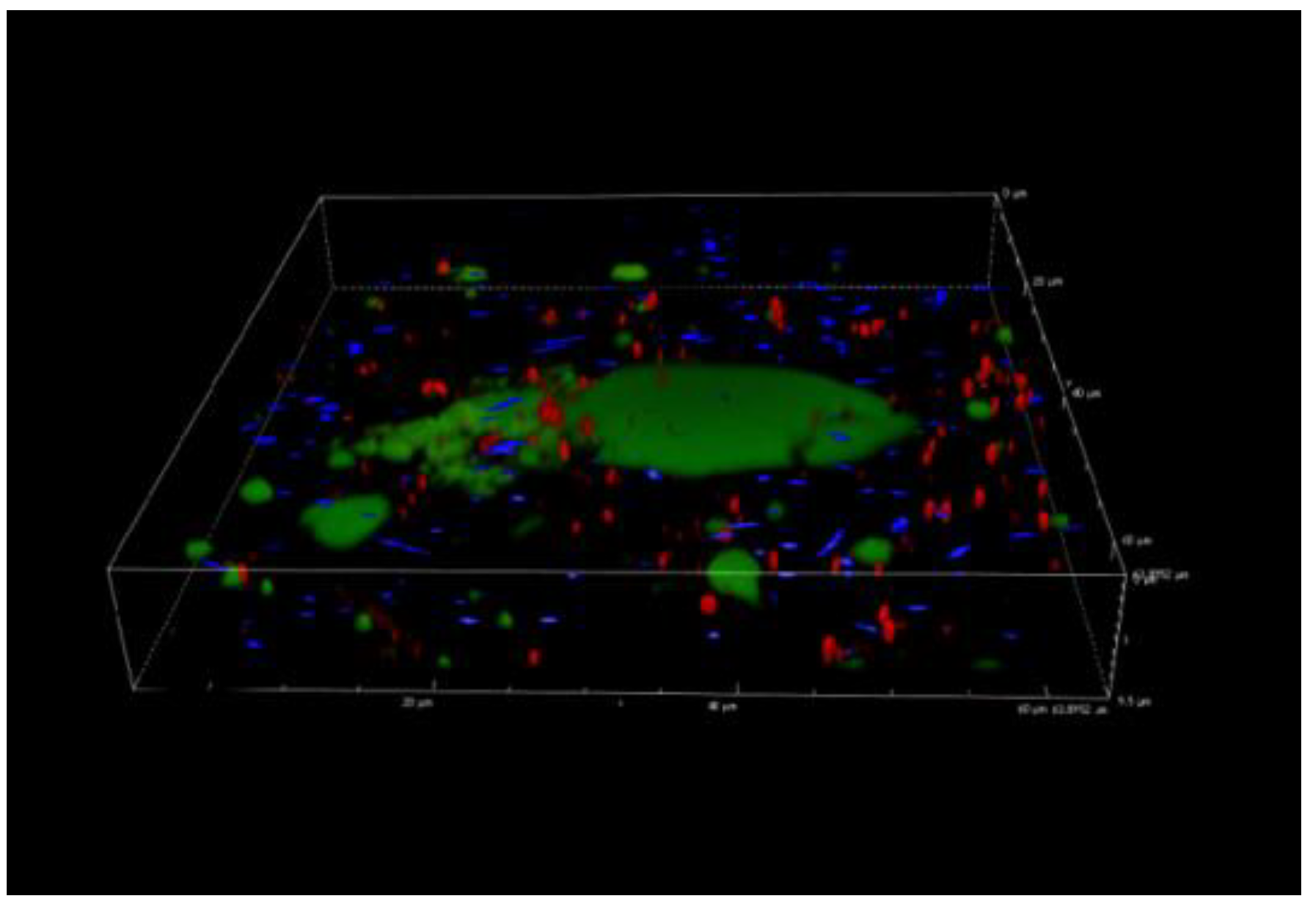
3.5. Confocal Laser Scanning Microscopy Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassinger, S.J.; Van Hoek, M.L. Biofilm architecture: An emerging synthetic biology target. Synth. Syst. Biotechnol. 2020, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Chaves-López, C.; Serio, A.; Anniballi, F.; Valbonetti, L.; Paparella, A. Effect of Origanum vulgare essential oil on biofilm formation and motility capacity of Pseudomonas fluorescens strains isolated from discolored Mozzarella cheese. J. Appl. Microbiol. 2018, 124, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.S.; Lopes, D.R.G.; Andre, C.; Silva, C.C.; Baglinière, F.; Vanetti, M.C.D. Multispecies biofilm formation by the contaminating microbiota in raw milk. Biofouling 2019, 35, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Di Ciccio, P.; Rubiola, S.; Grassi, M.A.; Civera, T.; Abbate, F.; Chiesa, F. Fate of Listeria monocytogenes in the Presence of Resident Cheese Microbiota on Common Packaging Materials. Front. Microbiol. 2020, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Serio, A.; Chaves-López, C.; Anniballi, F.; Auricchio, B.; Goffredo, E.; Goga, B.T.C.; Lista, F.R.; Fillo, S.; Paparella, A. Biofilm formation, pigment production and motility in Pseudomonas spp. isolated from the dairy industry. Food Control. 2018, 86, 241–248. [Google Scholar] [CrossRef]
- Reichler, S.J.; Martin, N.H.; Evanowski, R.L.; Kovac, J.; Wiedmann, M.; Orsi, R. A century of gray: A genomic locus found in 2 distinct Pseudomonas spp. is associated with historical and contemporary color defects in dairy products worldwide. J. Dairy Sci. 2019, 102, 5979–6000. [Google Scholar] [CrossRef] [PubMed]
- Nychas, G.-J.E.; Nychas, G.-J.E.; Koutsoumanis, K. Strain variability in biofilm formation: A food safety and quality perspective. Food Res. Int. 2020, 137, 109424. [Google Scholar] [CrossRef]
- Røder, H.L.; Sørensen, S.J.; Burmølle, M. Studying Bacterial Multispecies Biofilms: Where to Start? Trends Microbiol. 2016, 24, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Londero, A.; Costa, M.; Galli, L.; Brusa, V.; Linares, L.; Prieto, M.; Leotta, G. Characterization and subtyping of Listeria monocytogenes strains from butcher shops. LWT-Food Sci. Technol. 2019, 113, 108363. [Google Scholar] [CrossRef]
- De Carvalho, R.J.; De Souza, G.T.; Honório, V.G.; De Sousa, J.P.; Da Conceição, M.L.; Maganani, M.; De Souza, E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.B.D.S.; De Souza, E.L.; Scarano, J.O.A.; De Sousa, J.M.; Lira, M.C.; De Figueiredo, R.C.B.Q.; De Souza, E.L.; Magnani, M.; Souza, N.; Alcântara, J.O.; et al. Efficacy of using oregano essential oil and carvacrol to remove young and mature Staphylococcus aureus biofilms on food-contact surfaces of stainless steel. LWT 2018, 93, 293–299. [Google Scholar] [CrossRef]
- Abdallah, H.M.I.; Asaad, G.F.M.; Nada, S.A.; Taha, H.S.; Seif El-Nasr, M.M. Influence of extract derived in-vitro cell suspension cultures of Echinacea purpurea against some immunosuppressive effects. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1136–1143. [Google Scholar]
- Harimawan, A.; Ting, Y.P. Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion. Colloids Surf. B Biointerfaces 2016, 146, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Murphy, S.; Ralyea, R.; Wiedmann, M.; Boor, K. When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. J. Dairy Sci. 2011, 94, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Mendez, E.; Walker, D.K.; Vipham, J.; Trinetta, V. The use of a CDC biofilm reactor to grow multi-strain Listeria monocytogenes biofilm. Food Microbiol. 2020, 92, 103592. [Google Scholar] [CrossRef] [PubMed]
- Skowron, K.; Brożek, K.; Łukasik, M.; Wiktorczyk, N.; Korkus, J.; Gospodarek-Komkowska, E. Assessment of drug susceptibility and biofilm formation ability by clinical strains of Listeria monocytogenes. Disaster Emerg. Med. J. 2020, 5, 12–18. [Google Scholar] [CrossRef]
- Puga, C.H.; Dahdouh, E.; Sanjose, C.; Orgaz, B. Listeria monocytogenes Colonizes Pseudomonas fluorescens Biofilms and Induces Matrix Over-Production. Front. Microbiol. 2018, 9, 1706. [Google Scholar] [CrossRef] [PubMed]
- Chavant, P.; Gaillard-Martinie, B.; Talon, R.; Hébraud, M.; Bernardi, T. A new device for rapid evaluation of biofilm formation potential by bacteria. J. Microbiol. Methods 2007, 68, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Andreani, N.A.; Carraro, L.; Martino, M.E.; Fondi, M.; Fasolato, L.; Miotto, G.; Magro, M.; Vianello, F.; Cardazzo, B. A genomic and transcriptomic approach to investigate the blue pigment phenotype in Pseudomonas fluorescens. Int. J. Food Microbiol. 2015, 213, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, A.; Calzada, J.; Nuñez, M. The blue discoloration of fresh cheeses: A worldwide defect associated to specific contamination by Pseudomonas fluorescens. Food Control. 2018, 86, 359–366. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggio, F.; Rossi, C.; Chaves-López, C.; Serio, A.; Valbonetti, L.; Pomilio, F.; Chiavaroli, A.P.; Paparella, A. Interactions between L. monocytogenes and P. fluorescens in Dual-Species Biofilms under Simulated Dairy Processing Conditions. Proceedings 2021, 70, 80. https://doi.org/10.3390/foods_2020-07625
Maggio F, Rossi C, Chaves-López C, Serio A, Valbonetti L, Pomilio F, Chiavaroli AP, Paparella A. Interactions between L. monocytogenes and P. fluorescens in Dual-Species Biofilms under Simulated Dairy Processing Conditions. Proceedings. 2021; 70(1):80. https://doi.org/10.3390/foods_2020-07625
Chicago/Turabian StyleMaggio, Francesca, Chiara Rossi, Clemencia Chaves-López, Annalisa Serio, Luca Valbonetti, Francesco Pomilio, Alessio Pio Chiavaroli, and Antonello Paparella. 2021. "Interactions between L. monocytogenes and P. fluorescens in Dual-Species Biofilms under Simulated Dairy Processing Conditions" Proceedings 70, no. 1: 80. https://doi.org/10.3390/foods_2020-07625
APA StyleMaggio, F., Rossi, C., Chaves-López, C., Serio, A., Valbonetti, L., Pomilio, F., Chiavaroli, A. P., & Paparella, A. (2021). Interactions between L. monocytogenes and P. fluorescens in Dual-Species Biofilms under Simulated Dairy Processing Conditions. Proceedings, 70(1), 80. https://doi.org/10.3390/foods_2020-07625








