Abstract
Spirulina platensis, the most popular microalgae species known for its high protein content and bioactive compounds such as phycocyanin and allophycocyanin, has been studied for cheese fortification. Incorporation of spirulina in dairy products poses major sensorial challenges due to its characteristic odor and its insolubility in food formulation, thus limiting consumer acceptance. The main objective was the production of a novel spread cheese fortified with spirulina, so powdered spirulina was added at different concentrations (0.25, 0.5, and 1%), and the effect on physicochemical, microbiological, and sensory characteristics was assessed. Cheese samples were examined for pH, fat (Gerber-Van Gulik method), salt (Volhard method), protein (Kjeldahl), and moisture content by drying to constant weight at 102 ± 1 °C. Cheeses were also assessed organoleptically by five experienced panelists. Generally, the addition of pirulina slightly increased the protein content and affected the color of the cheeses. The cheeses achieved a good microbiological profile and were all characterized as acceptable for consumption by the panelists. However, the cheeses with 0.25 and 0.5% spirulina were mostly preferred by the evaluation panel due to the less intense characteristic odor and taste of spirulina. We conclude that it is possible to produce an acceptable spread cheese with the addition of spirulina without significant changes in the cheese production line.
1. Introduction
Milk and dairy products have an important role in the human diet due to their nutritional benefits from proteins, minerals, and vitamins. Especially in Greece, dairy products are consumed massively and are an important part of the daily Greek diet. Recently, food scientists have tried the fortification of dairy products using natural products to ameliorate the overall dietary intake of foods with minimal effects [1]. Microalgae are known to be rich in proteins, amino acids, vitamins, and various minerals, as well as polysaccharides, sterols, and fatty acids. They have great potential to be used as fortification products in dairy as they contain various macro- components (polysaccharides and sulphated polysaccharides as stabilizer) and micro-components (polyunsaturated fatty acids as bioactive compounds and pigments as a coloring agent) posing important functional characteristics [2]. Arthrospira (Spirulina) platensis, the most popular microalgae species known for its high protein content and bioactive compounds has been studied as such potential natural resource for dairy products fortification [2]. Although the technological impacts of spirulina in fermented milks has been studied before, the effect on the nutritional and sensorial qualities of soft cheese has not been addressed. Also, incorporation of dried spirulina in dairy products poses major sensorial challenges due to its characteristic odor and its insolubility in food formulation thus limiting consumer acceptance. The main objectives of the current study were to produce a novel functional soft cheese fortified with powdered spirulina, to determine the concentration of Arthrospira (Spirulina) platensis that can be added to the product and to evaluate its effects on the physicochemical, textural, and sensorial characteristics of the cheese produced.
2. Materials and Methods
2.1. Production of Spirulina Enriched Fresh Soft Cheese
24 kg of ewe’s milk (fat: 5.43, protein: 5.20, lactose: 4.61, total solids: 16.05) was collected after milking, cloth filtered to remove contaminants and external material, heated up to 85 °C, and then cooled down to 35 °C where yogurt culture was added and divided into equal portions (6 kg each) in stainless steel buckets with a lid. In each bucket 1 mL Kg−1 of milk CaCl2 (40% w/v) and liquid commercial rennin (1 mL Kg−1) were added. Subsequently the samples were placed in a chamber at 20 °C for 24 h. After 24 h the curd was removed from each container and placed into perforated fabric called ‘tsandila’ for cheese whey removal. The cheese curd of each sample was left to drain into the fabric bags in a chamber at 12 °C for 48 h. Finally, the samples were removed from the fabric bags, weighed, and placed in special storage containers and salt (type 2, 1 g kg−1 of cheese) and spirulina in powder form (0.25, 0.5, and 1 g kg−1) were added.
2.2. Physicochemical, Microbiological, and Sensory Analysis of Fresh Soft Cheese Enriched with Powered Spirulina
The milk for the cheesemaking was analyzed for physicochemical parameters, i.e., fat, protein, lactose, total solid by Milko-Scan, model 6000 (Foss Electric, Hillerød, Denmark). Milk pH was measured directly with a pH meter (Micro pH 2002; Crison, Barcelona, Spain). Microbiological evaluation was carried out by assessing the total viable counts (TVC) using the Bactoscan FC (Foss Electric, Hillerød, Denmark).
Cheese samples were examined for pH electrometrically (Micro pH 2002; Crison, Barcelona, Spain) and were analyzed for their fat according to the Gerber-Van Gulik method, salt according to the modified Volhard method [3] and moisture content by drying to constant weight at 102 ± 1 °C [4].
On each sampling date, ten-gram portions of each cheese sample were added to 90 mL with sterilized Ringer solution ¼ strength and mixed with a stomacher (Bagmixer 400, Model VW, Interscience) for 120 s at room temperature. Subsequent dilutions were made in sterilized Ringer’s solution ¼ strength. Viable counts for staphylococci, lactic acid bacteria, lactic cocci, molds and yeasts, coliforms, and enterobacteria were performed in duplicate. 1-mL or 0.1-mL samples of appropriate dilutions were poured or spread on total or selective agar plates for each species and according to instructions given by the manufacturer. Unless otherwise stated, all media and supplements were purchased from Neogen Culture Media (Heywood, UK).
Coliform counts were enumerated on violet red bile agar after incubation at 30 °C for 24 h, and total Enterobacteriaceae were enumerated on violet red bile glucose agar after incubation at 37 °C for 24 h. Total mesophilic lactic acid bacteria (LAB) were enumerated on de Man, Rogosa, Sharpe (MRS) agar, incubated at 37 °C for 72 h under aerobic conditions. Mesophilic cocci were enumerated on M17 agar, incubated at 30 °C for 48–72 h. Total staphylococci were enumerated on Baird Parker agar base with egg yolk tellurite (BP), incubated at 37 °C for 48 h; yeasts and molds were enumerated on rose Bengal chloramphenicol agar, incubated at 25 °C for 5 days.
A three-way repeated measures analysis was applied to the values of each produced cheese and the results are presented as mean values ± standard deviation. The significance of differences in the means of various groups was checked by One-way Analysis of Variance (ANOVA) at the 5% level of significance. p-values below 0.05 were considered significant.
A consumer acceptance test was conducted by five experts (25–55 years old) concerning attributes of dairy—sour taste, microalgae odor and taste, sweetness, bitterness, dairy flavor, crumbly texture, smoothness, color, and overall acceptability. Assessors were non-smokers and they were familiar with the consumption of dairy and fermented milk products [5].
3. Results & Discussion
3.1. Cheese Production and Physicochemical Analysis
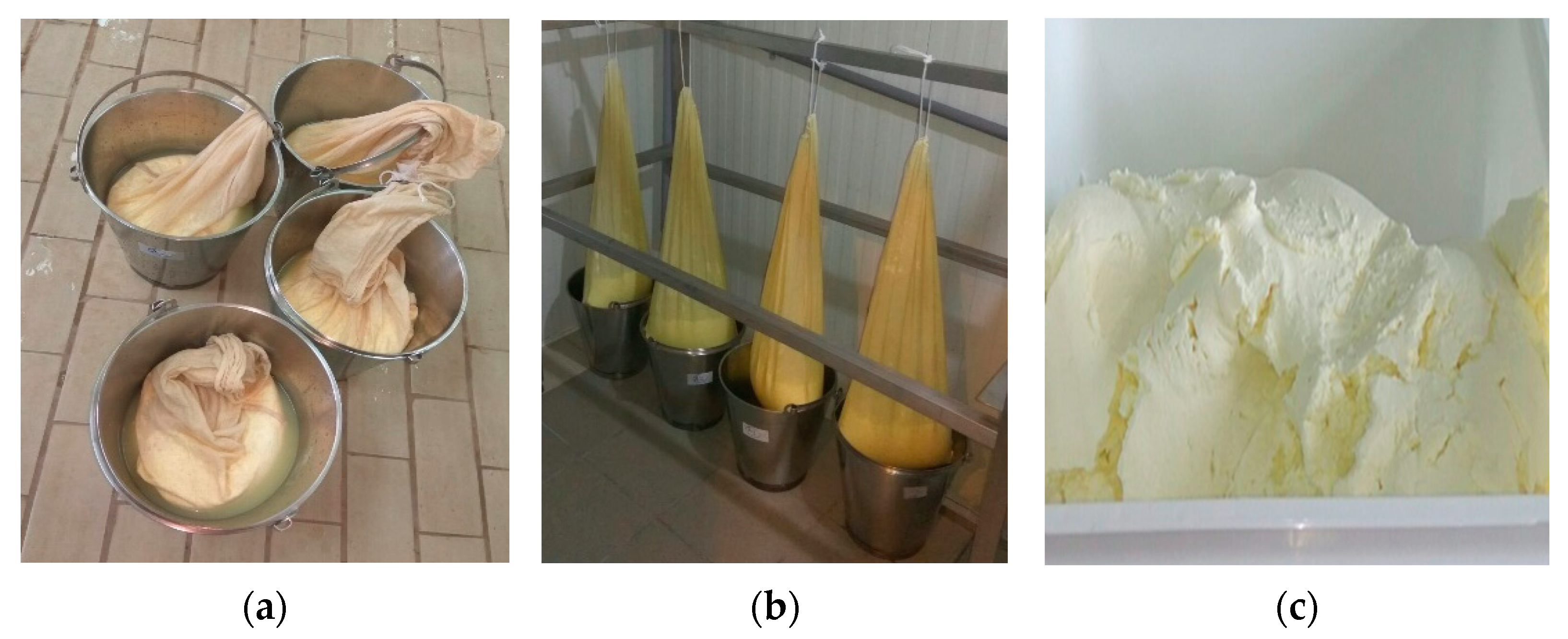
Galotyri is a fresh traditional Greek soft cheese. It is prepared using full fat sheep milk either raw or pasteurized. In the present study ewe’s milk was applied without pasteurization to maintain its aromatic and nutritional characteristics as the initial samples were submitted to cheese making instantly after milk collection. Milk was collected immediately after milking, filtered and heat treated at 85 °C, cooled down to 35 °C, and yogurt culture was added; the mixture was divided into equal portions (6 kg each). The yield of milk in cheese is estimated at 40% as from the 6kg of ewe’s milk we receive 2.4 kg ± 0.4 of cheese. In each bucket 1 mL/Kg of cheese of CaCl2 (40% w/v) and liquid rennin were added. In Figure 1 are illustrated the stages of production until the final cheese curd was retrieved (Figure 1c). To produce galotyri with incorporated spirulina, the samples were removed from the fabric bags, weighed, and placed in special storage containers (Figure 1c), salted (type 2, 1 g kg−1 of cheese) and mixed with powdered spirulina (0.25, 0.5, and 1 g kg−1) (Figure 2).

Figure 1.
Manufacture stages of galotyri fresh cheese. (a) Samples removed from their initial containers and placed into perforated fabric called ‘tsandila’ targeting cheese whey removal; (b) the cheese curd of each sample is left to drain into fabric bags in a chamber at 12 °C for 48 h; (c) cheese curd retrieved after drainage.

Figure 2.
(a) Addition of powder spirulina in fresh cheese; (b) fresh cheese type-Galotyri with incorporated Arthrospira (Spirulina) platensis.
The chemical composition of galotyri was determined during storage at 4 °C after two days of production (Table 1). Products of microalgae origin have been widely used as a high-protein supplements especially in human nutrition, as well as for nutraceutical purposes. In the present study a slight rise in protein content was observed as the content of incorporated spirulina was incorporated in higher amounts. This result was mostly expected as Arthrospira (Spirulina) platensis is one of the most nutritious microalgae known, containing a wide capacity of essential amino acids and protein content of up to 70% of its dry biomass [6].

Table 1.
Chemical composition of fresh cheese so named ‘galotyri’ determined two days after the addition of salt and powered spirulina.
3.2. Cheese Microbiological Profile
Microorganisms in fermented dairy impose a significant impact on physicochemical and organoleptic characteristics of the final product [4,7]. Moreover, microorganisms as well as their bioactive metabolites produced in fermented dairy can be responsible for immunomodulatory and antiviral effects when consumed in adequate amounts [8]. Likewise, microbial stability has a crucial role in the maintenance of a dairy product’s bioactivity and original character [9]. In the present study, the different concentrations (0.25, 0.5, 1%) of incorporated spirulina did not affect the final microbiological profile of produced cheeses (Table 2). The usual storage time of microbiologically accepted fresh cheeses such as galotyri is up to 30 days after production. Likewise, in the present study, we observed the increase in population of possible spoilage microorganism such as yeast and molds after 60 days of storage in all cheese samples.

Table 2.
Microbiological profile of galotyri during cold storage at 4 °C for 60 days.
3.3. Sensory Evaluation: Consumers Acceptance Test
Spirulina-enriched fresh cheeses were produced by the addition of powdered spirulina in different concentrations (Figure 2a). Spirulina, like most microalgae, is known to possess an undesirable odor. As a matter of fact, scientific researchers have investigated methods to reduce the algae-related odor of spirulina using activated charcoal absorption, heating, lysozyme enzymatic hydrolysis, β-cyclodextrin inclusion, fermentation, and solvent extraction. Fermentation and ethanol extraction have been proved to be the most effective methods for the reduction of the algae-like odor of spirulina [10]. Likewise, in the present study an algae-like odor was detected in the cheese samples, but according to the intensity of powdered spirulina. Specifically, the cheeses with 0.25% and 0.5% incorporated spirulina were mostly preferred by the panelists as they possessed a less intense odor and taste of spirulina. In general, all cheese samples received high preference scores and were characterized as acceptable for consumption by the expert evaluation panel.
4. Conclusions
Microalgae biomass is considered a sustainable source of proteins that can meet the growing global demand for these biomolecules. Spirulina is one of the most nutritious microalgae known and can reach a protein content of up to 70% of the dry biomass. Spirulina has great potential to be used as a functional fortification ingredient in dairy. Likewise, in the present study the protein fortification, microbiological stability, and consumers’ acceptance were verified in the production of a novel fresh cheese containing powdered spirulina, providing a nutritional product, while the original recipe and character of traditional galotyri cheese were preserved.
Author Contributions
Conceptualization, L.B. and G.M.; methodology, L.B., G.M.; software, M.M.; validation, G.K., A.T. and L.B.; formal analysis, E.P., E.K.; investigation, L.B., G.M. & A.T.; resources, L.B.; data curation, E.P. and A.T.; writing—original draft preparation, A.T.; writing—review and editing, L.B.; visualization, L.B., G.M. & A.T.; supervision, L.B.; project administration, L.B.; funding acquisition, L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research (MIS number: 5040303, project title: Innovative cheese products with microalgae) was funded by the action ‘Support of research, development and innovation projects- RIS3 Agri-foods’ and was co-financed by the European Union (European Regional Development Fund) and Greece, under the “Operational Program Western Greece 2014–2020” of the National Strategic Reference Framework.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, Y.; Kilicli, M.; Toker, O.S.; Konar, N.; Palabiyik, I.; Tamtürk, F. Using spray-dried microalgae in ice cream formulation as a natural colorant: Effect on physicochemical and functional properties. Algal Res. 2020, 47, 101811. [Google Scholar] [CrossRef]
- Fernandez, A.; Kosikowski, F.V. Low Moisture Mozzarella Cheese from Whole Milk Retentates of Ultrafiltration. J. Dairy Sci. 1986, 69, 2011–2017. [Google Scholar] [CrossRef]
- Terpou, A.; Bosnea, L.; Kanellaki, M.; Plessas, S.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A. Growth Capacity of a Novel Potential Probiotic Lactobacillus paracasei K5 Strain Incorporated in Industrial White Brined Cheese as an Adjunct Culture. J. Food Sci. 2018, 83, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT 2019, 105, 242–249. [Google Scholar] [CrossRef]
- da Fontoura Prates, D.; Duarte, J.H.; Vendruscolo, R.G.; Wagner, R.; Ballus, C.A.; da Silva Oliveira, W.; Godoy, H.T.; Barcia, M.T.; de Morais, M.G.; Radmann, E.M.; et al. Role of light emitting diode (LED) wavelengths on increase of protein productivity and free amino acid profile of Spirulina sp. cultures. Bioresour. Technol. 2020, 306, 123184. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Gialleli, A.-I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT Food Sci. Technol. 2017, 79, 616–624. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Zawawi, N.; Abdull Razis, A.F.; Bakar, J.; Zarei, M. Antiviral activity of fermented foods and their probiotics bacteria towards respiratory and alimentary tracts viruses. Food Control 2021, 127, 108140. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhang, X.; Zheng, J.-H.; Ren, D.-F.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).