Solid Dispersions as a Technological Strategy to Improve the Bio-Performance of Antiparasitic Drugs with Limited Solubility †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. BZL Extraction Procedure
2.3. SDs Preparation
2.4. Physico-Chemical Carachterization
2.4.1. X-ray Diffraction
2.4.2. Scanning Electron Microscopy
2.4.3. Phase and Saturation Solubility Studies
2.5. Dissolution Test
3. Results
3.1. Physical-Chemical Carachterization
3.1.1. X-ray Diffraction
3.1.2. Scanning Electron Microscopy
3.1.3. Phase and Saturation Solubility Studies
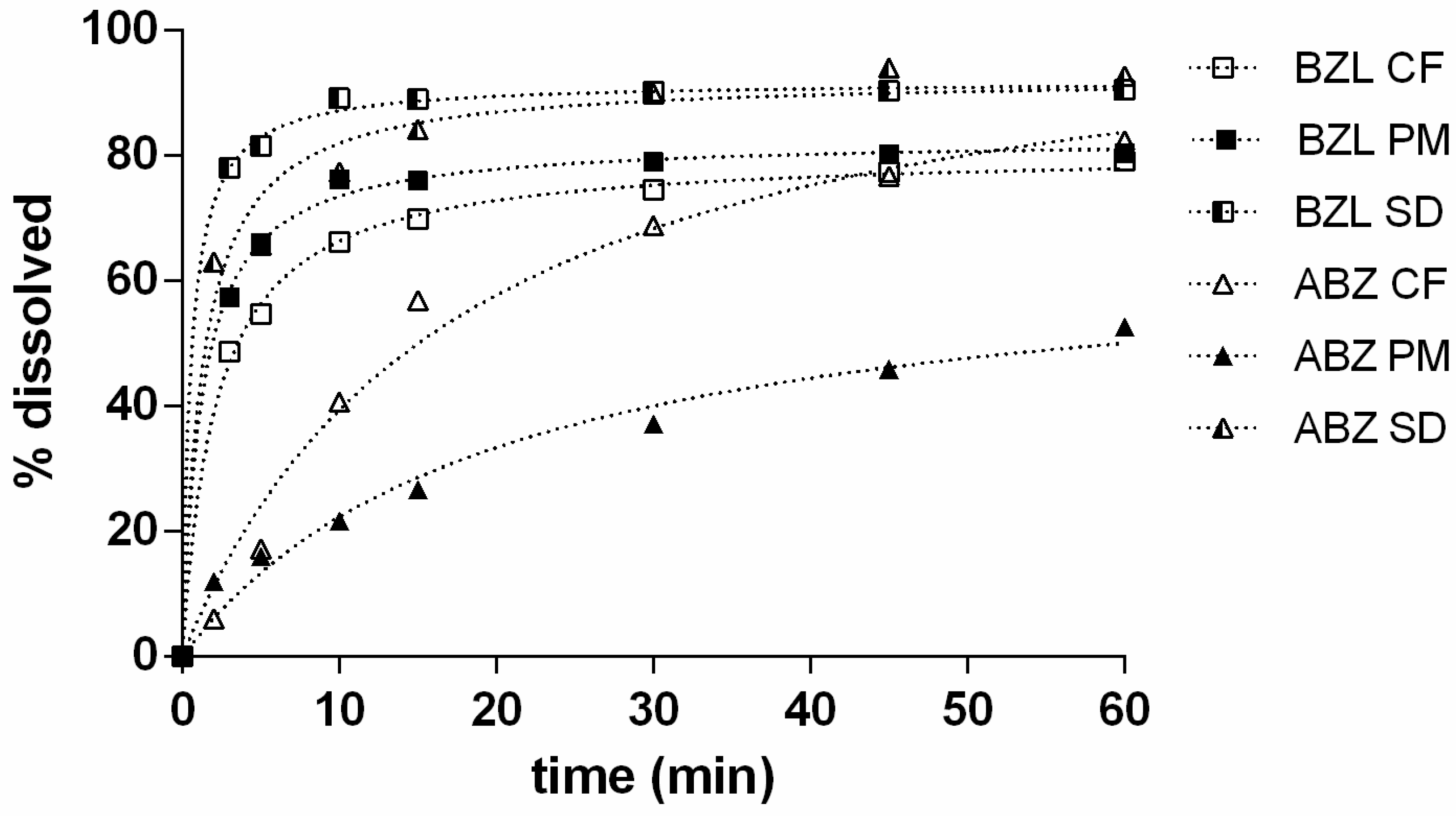
3.2. Dissolution Test
4. Discussion
4.1. Physico-Chemical Carachterization
4.1.1. X-ray Diffraction
4.1.2. Scanning Electron Microscopy
4.1.3. Phase and Saturation Solubility
4.2. Dissolution Test
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Dayan, A.D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003, 86, 141–159. [Google Scholar] [CrossRef]
- Bahia, M.T.; Diniz Lde, F.; Mosqueira, V.C. Therapeutical approaches under investigation for treatment of Chagas disease. Expert Opin. Investig. Drugs 2014, 23, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y. Pharmaceutical Technologies for Enhancing Oral Bioavailability of Poorly Soluble Drugs. J. Bioequiv. Bioavailab. 2010, 2. [Google Scholar] [CrossRef]
- Castro, S.G.; Bruni, S.S.; Lanusse, C.E.; Allemandi, D.A.; Palma, S.D. Improved Albendazole Dissolution Rate in Pluronic 188 Solid Dispersions. AAPS PharmSciTech 2010, 11, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- Lima, Á.A.N.; Soares-Sobrinho, J.L.; Silva, J.L.; Corrêa-Júnior, R.A.C.; Lyra, M.A.M.; Santos, F.L.A.; Oliveira, B.G.; Hernandes, M.Z.; Rolim, L.A.; Rolim-Neto, P.J. The Use of Solid Dispersion Systems in Hydrophilic Carriers to Increase Benznidazole Solubility. J. Pharm. Sci. 2011, 100, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Leonardi, D.; Lamas, M.C. Promising applications in drug delivery systems of a novel β-cyclodextrin derivative obtained by green synthesis. Bioorg. Med. Chem. 2016, 26, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Soares-Sobrinho, J.L.; Santos, F.L.A.; Lyra, M.A.M.; Alves, L.D.S.; Rolim, L.A.; Lima, A.A.N.; Nunes, L.C.C.; Soares, M.F.R.; Rolim-Neto, P.J.; Torres-Labandeira, J.J. Benznidazole drug delivery by binary and multicomponent inclusion complexes using cyclodextrins and polymers. Carbohydr. Polym. 2012, 89, 323–330. [Google Scholar] [CrossRef]
- Sobrinho, J.L.S.; Soares, M.F.; Labandeira, J.J.T.; Alves, L.D.S.; Rolim Neto, P.J. Improving the solubility of the antichagasic drug benznidazole through formation of inclusion complexes with cyclodextrins. Quim. Nova 2011, 34, 1534–1538. [Google Scholar] [CrossRef]
- Vogt, M.; Kunath, K.; Dressman, J.B. Dissolution improvement of four poorly water soluble drugs by cogrinding with commonly used excipients. Eur. J. Pharm Biopharm. 2008, 68, 330–337. [Google Scholar] [CrossRef]
- Priotti, J.; Codina, A.V.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Albendazole Microcrystal Formulations Based on Chitosan and Cellulose Derivatives: Physicochemical Characterization and In Vitro Parasiticidal Activity in Trichinella spiralis Adult Worms. AAPS PharmSciTech 2017, 18, 947–956. [Google Scholar] [CrossRef]
- Maximiano, F.P.; de Paula, L.M.; Figueiredo, V.P.; de Andrade, I.M.; Talvani, A.; Sá-Barreto, L.C.; Bahia, M.T.; Cunha-Filho, M.S.S. Benznidazole microcrystal preparation by solvent change precipitation and in vivo evaluation in the treatment of Chagas disease. Eur. J. Pharm. Biopharm. 2011, 78, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Seremeta, K.P.; Arrúa, E.C.; Okulik, N.B.; Salomon, C.J. Development and characterization of benznidazole nano- and microparticles: A new tool for pediatric treatment of Chagas disease? Colloid Surf. B 2019, 177, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.J.; Llabot, J.M.; Sánchez Bruni, S.; Allemandi, D.; Palma, S.D. Self-dispersible nanocrystals of albendazole produced by high pressure homogenization and spray-drying. Drug Dev. Ind. Pharm. 2016, 42, 1564–1570. [Google Scholar] [CrossRef]
- Mukherjee, T.; Plakogiannis, F.M. Development and oral bioavailability assessment of a supersaturated self-microemulsifying drug delivery system (SMEDDS) of albendazole. J. Pharm. Pharmacol. 2010, 62, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Mazzeti, A.L.; Oliveira, L.T.; Gonçalves, K.R.; Schaun, G.C.; Mosqueira, V.C.F.; Bahia, M.T. Benznidazole self-emulsifying delivery system: A novel alternative dosage form for Chagas disease treatment. Eur. J. Pharm. Sci. 2020, 145, 105234. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Obi, N. Studies on Absorption of Eutectic Mixture. I. A Comparison of the Behavior of Eutectic Mixture of Sulfathiazole and that of Ordinary Sulfathiazole in Man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000, 18, 412–420. [Google Scholar] [CrossRef]
- García, M.C.; Ponce, N.E.; Sanmarco, L.M.; Manzo, R.H.; Jimenez-Kairuz, A.F.; Aoki, M.P. Clomipramine and Benznidazole Act Synergistically and Ameliorate the Outcome of Experimental Chagas Disease. Antimicrob. Agents Chemother. 2016, 60, 3700–3708. [Google Scholar] [CrossRef]
- Fernández-Colino, A.; Bermudez, J.M.; Arias, F.; Quinteros, D.; Gonzo, E. Development of a mechanism and an accurate and simple mathematical model for the description of drug release: Application to a relevant example of acetazolamide-controlled release from a bio-inspired elastin-based hydrogel. Mat. Sci. Eng. C Mater. 2016, 61, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.I.; Villegas, M.; Cid, A.G.; Parentis, M.L.; Gonzo, E.E.; Bermúdez, J.M. Validation of kinetic modeling of progesterone release from polymeric membranes. Asian J. Pharm. Sci. 2018, 13, 54–62. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- El-Badry, M.; Hassan, M.A.; Ibrahim, M.A.; Elsaghir, H. Performance of poloxamer 407 as hydrophilic carrier on the binary mixtures with nimesulide. Farmacia 2013, 61, 1137–1150. [Google Scholar]
- Simonazzi, A.; Davies, C.; Cid, A.G.; Gonzo, E.; Parada, L.; Bermúdez, J.M. Preparation and characterization of Poloxamer 407 solid dispersions as an alternative strategy to improve benznidazole bioperformance. J. Pharm. Sci. 2018, 107, 2829–2836. [Google Scholar] [CrossRef] [PubMed]


| Sample | IDR (%*min−1) | t80% (min) | DE (%) |
|---|---|---|---|
| ABZ CF | 6.74 | 40.31 | 65.11 |
| ABZ PM | 3.34 | NR | 35.93 |
| ABZ SD | 71.62 | 1.69 | 84.91 |
| BZL CF | 37.24 | 239.21 | 64.98 |
| BZL PM | 66.03 | 46.45 | 71.58 |
| BZL SD | 170.23 | 3.64 | 85.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, S.N.; Cid, A.G.; Romero, A.I.; Villegas, M.; Briones Nieva, C.A.; Gonzo, E.E.; Bermúdez, J.M. Solid Dispersions as a Technological Strategy to Improve the Bio-Performance of Antiparasitic Drugs with Limited Solubility. Proceedings 2021, 78, 13. https://doi.org/10.3390/IECP2020-08686
Campos SN, Cid AG, Romero AI, Villegas M, Briones Nieva CA, Gonzo EE, Bermúdez JM. Solid Dispersions as a Technological Strategy to Improve the Bio-Performance of Antiparasitic Drugs with Limited Solubility. Proceedings. 2021; 78(1):13. https://doi.org/10.3390/IECP2020-08686
Chicago/Turabian StyleCampos, Santiago N., Alicia G. Cid, Analía I. Romero, Mercedes Villegas, Cintia A. Briones Nieva, Elio E. Gonzo, and José M. Bermúdez. 2021. "Solid Dispersions as a Technological Strategy to Improve the Bio-Performance of Antiparasitic Drugs with Limited Solubility" Proceedings 78, no. 1: 13. https://doi.org/10.3390/IECP2020-08686
APA StyleCampos, S. N., Cid, A. G., Romero, A. I., Villegas, M., Briones Nieva, C. A., Gonzo, E. E., & Bermúdez, J. M. (2021). Solid Dispersions as a Technological Strategy to Improve the Bio-Performance of Antiparasitic Drugs with Limited Solubility. Proceedings, 78(1), 13. https://doi.org/10.3390/IECP2020-08686





