Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials †
Abstract
:1. Introduction
2. Experiments
2.1. Materials and Methods
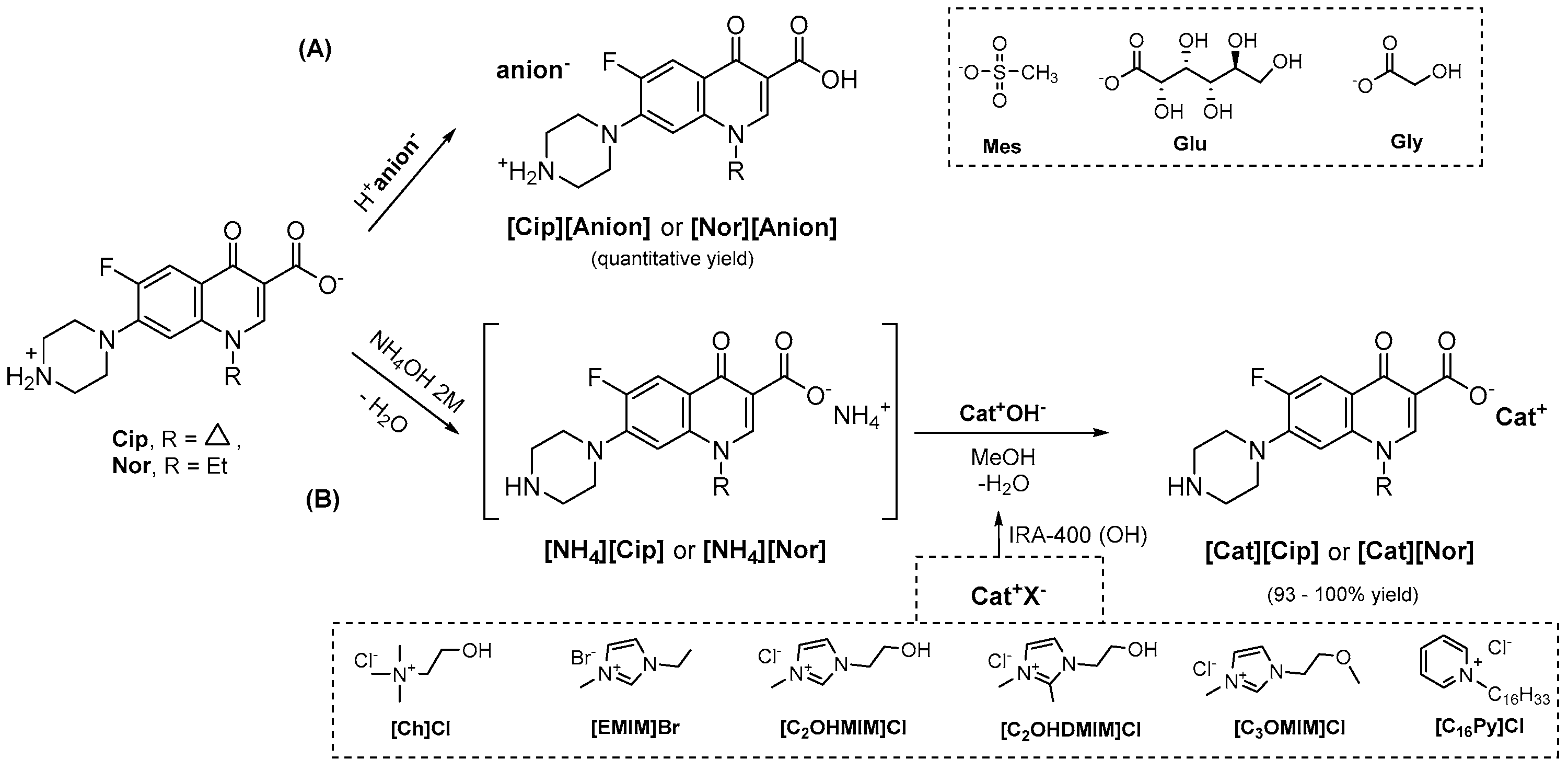
2.1.1. General Procedure for the Preparation of Cationic FQ-OSILs
2.1.2. General Procedure for the Preparation of Anionic FQ-OSILs
2.1.3. Determination of the Solubility in Water
2.1.4. Cytotoxicity of Compounds
2.1.5. Antimicrobial Activities
2.1.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis
3.2. Spectroscopic Characterization
3.3. Thermal Analysis
3.4. Water Solubility Studies
3.5. Antimicrobial Activity Studies
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| API–OSILs | Active Pharmaceutical Ingredient Organic Salts and Ionic Liquids |
| C16Py | cetylpyridinium |
| C2OHDMIM | 1-(2-hydroxyethyl)-2,3-dimethylimidazolium |
| C2OHMIM | 1-(2-hydroxyethyl)-3-methylimidazolium |
| C3OMIM | 1-(2-methoxyethyl)-3-methylimidazolium |
| Ch | choline |
| Cip | ciprofloxacin |
| DSMZ | Deutsche Sammlung von Mikroorganismen und Zellkulturen |
| EMIM | 1-ethyl-3-methylimidazolium |
| FQ | fluoroquinolone |
| FQ-OSILs | fluoroquinolone-based organic salts and ionic liquids |
| IC50 | half maximal inhibitory concentration |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| Nor | norfloxacin |
| OSIL | Organic Salt and Ionic Liquid |
| RDIC50 | Relative Decrease in Inhibitory Concentration for 50% bacterial growth |
| RTILs | Room Temperature Ionic Liquids |
| Tg | glass transition temperature |
| Tm | melting temperature |
References
- Holmes, B.; Brogden, R.N.; Richards, D.M. Norfloxacin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1985, 30, 482–513. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enz. Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Emmerson, A.M.; Jones, A.M. The quinolones: Decades of development and use. J. Antimicrob. Chemother. 2003, 51, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Variankaval, N.; Cote, A.S.; Doherty, M.F. From form to function: Crystallization of active pharmaceutical ingredients. AIChE J. 2008, 54, 1682–1688. [Google Scholar] [CrossRef]
- Nikaido, H.; Thanassi, D.G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: Tetracyclines and fluoroquinolones as examples. Antimicrob. Agents Chemother. 1993, 37, 1393–1399. [Google Scholar] [CrossRef]
- Yu, X.; Zipp, G.; Davidson, G.W.R., III. The effect of temperature and pH on the solubility of quinolone compounds: Estimation of heat of fusion. Pharm. Res. 1994, 11, 522–527. [Google Scholar] [CrossRef]
- Varanda, F.; Pratas de Melo, M.J.; Caço, A.I.; Dohrn, R.; Makrydaki, F.A.; Voutsas, E.; Tassios, D.; Marrucho, I.M. Solubility of antibiotics in different solvents. 1. Hydrochloride forms of tetracycline, moxifloxacin, and ciprofloxacin. Ind. Eng. Chem. Res. 2006, 45, 6368–6374. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Marrucho, I.; Araújo, J.; da Ponte, M.N.; Prudêncio, C.; Noronha, J.P.; Petrovski, Z. Development of Novel Ionic Liquids-APIs based on Ampicillin derivatives. Med. Chem. Comm. 2012, 3, 494–497. [Google Scholar] [CrossRef]
- Florindo, C.; Araujo, J.M.M.; Alves, F.; Matos, C.; Ferraz, R.; Prudencio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.; Rebelo, L.P.N.; et al. Evaluation of solubility and partition properties of ampicillin-based ionic liquids. Int. J. Pharm. 2013, 456, 553–559. [Google Scholar] [CrossRef]
- Ferraz, R.; Teixeira, V.; Rodrigues, D.; Fernandes, R.; Prudencio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Antibacterial activity of Ionic Liquids based on ampicillin against resistant bacteria. RSC Adv. 2014, 4, 4301–4307. [Google Scholar] [CrossRef]
- Ferraz, R.; Silva, D.; Dias, A.R.; Dias, V.; Santos, M.M.; Pinheiro, L.; Prudêncio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts based on Penicillin G and Amoxicillin hydrolysate derivatives against Resistant Bacteria. Pharmaceutics 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Costa, A.; Matos, C.; Nunes, S.L.; Matias, A.N.; Duarte, C.M.M.; Rebelo, L.P.N.; Branco, L.C.; Marrucho, I.M. Novel organic salts based on fluoroquinolone drugs: Synthesis, bioavailability and toxicological profiles. Int. J. Pharm. 2014, 469, 179–189. [Google Scholar] [CrossRef]
- Santos, M.M.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Branco, L.C. Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones. Pharmaceutics 2020, 12, 694. [Google Scholar] [CrossRef]
- Wu, H.; Deng, Z.; Zhou, B.; Qi, M.; Hong, M.; Ren, G. Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. J. Mol. Liq. 2019, 283, 399–409. [Google Scholar] [CrossRef]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic Liquids as Potential and Synergistic Permeation Enhancers for Transdermal Drug Delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef]
- Reddy, J.S.; Ganesh, S.V.; Nagalapalli, R.; Dandela, R.; Solomon, K.A.; Kumar, K.A.; Goud, N.R.; Nangia, A. Fluoroquinolone salts with carboxylic acids. J. Pharm. Sci. 2011, 100, 3160–3176. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Roussel, P.; Perlovich, G.L. Diversity of crystal structures and physicochemical properties of ciprofloxacin and norfloxacin salts with fumaric acid. CrystEngComm 2018, 20, 755–767. [Google Scholar] [CrossRef]
- Surov, A.O.; Manin, A.N.; Voronin, A.P.; Drozd, K.V.; Simagina, A.A.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical salts of ciprofloxacin with dicarboxylic acids. Eur. J. Pharm. Sci. 2015, 77, 112–121. [Google Scholar] [CrossRef]
- Surov, A.O.; Churakov, A.V.; Perlovich, G.L. Three Polymorphic Forms of Ciprofloxacin Maleate: Formation Pathways, Crystal Structures, Calculations, and Thermodynamic Stability Aspects. Cryst. Growth Des. 2016, 16, 6556–6567. [Google Scholar] [CrossRef]
- Paluch, K.J.; McCabe, T.; Müller-Bunz, H.; Corrigan, O.I.; Healy, A.M.; Tajber, L. Formation and physicochemical properties of crystalline and amorphous salts with different stoichiometries formed between ciprofloxacin and succinic acid. Mol. Pharm. 2013, 10, 3640–3654. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.P.; Ghosh, S.; Khan, H.; Devarapalli, R.; Reddy, C.M. Drug–drug salt forms of ciprofloxacin with diflunisal and indoprofen. CrystEngComm 2014, 16, 7393–7396. [Google Scholar] [CrossRef]
- Surov, A.O.; Vasilev, N.A.; Churakov, A.V.; Stroh, J.; Emmerling, F.; Perlovich, G.L. Solid Forms of Ciprofloxacin Salicylate: Polymorphism, Formation Pathways, and Thermodynamic Stability. Cryst. Growth Des. 2019, 19, 2979–2990. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Lesnikov, M.K.; Atuchin, V.V. Two salts and the salt cocrystal of ciprofloxacin with thiobarbituric and barbituric acids: The structure and properties. J. Phys. Org. Chem. 2018, 31, e3773. [Google Scholar] [CrossRef]
- Mesallati, H.; Conroy, D.; Hudson, S.; Tajber, L. Preparation and Characterization of Amorphous Ciprofloxacin-Amino Acid Salts. Eur. J. Pharm. Biopharm. 2017, 121, 73–89. [Google Scholar] [CrossRef]
- Mesallati, H.; Umerska, A.; Paluch, K.J.; Tajber, L. Amorphous Polymeric Drug Salts as Ionic Solid Dispersion Forms of Ciprofloxacin. Mol. Pharm. 2017, 14, 2209–2223. [Google Scholar] [CrossRef]
- Mesallati, H.; Umerska, A.; Tajber, L. Fluoroquinolone Amorphous Polymeric Salts and Dispersions for Veterinary Uses. Pharmaceutics 2019, 11, 268. [Google Scholar] [CrossRef]

| Compound | Physical State | Tm/°C | Tg/°C |
|---|---|---|---|
| Cip | White solid | 322.0 | - |
| [Cip][Mes] | Pale yellow solid | 145.5 | 125.2 |
| [Cip][Glu] | Pale yellow solid | 146 dec. | 122.9 |
| [Cip][Gly] | White solid | 153.7 | - |
| [Ch][Cip] | Pale yellow solid | 111.2 | - |
| [EMIM][Cip] | White solid | 92.9 | 36.7 |
| [C2OHMIM][Cip] | White solid | 111.6 | 49.5 |
| [C2OHDMIM][Cip] | White solid | 197.5 | - |
| [C3OMIM][Cip] | White solid | 112.4 | 46.4 |
| [C16Py][Cip] | Orange viscous liquid | - | -10.7 |
| Nor | White solid | 217.0 | |
| [Nor][Mes] | Pale yellow solid | 168.2 | - |
| [Nor][Glu] | Yellow viscous liquid | - | 1.8 |
| [Nor][Gly] | White solid | 115.3 | 91.6 |
| [Ch][Nor] | Pale yellow solid | 94.5 | 54.8 |
| [EMIM][Nor] | Yellow solid | 119.9 | 64.6 |
| [C2OHMIM][Nor] | Yellow viscous liquid | - | 41.1 |
| [C2OHDMIM][Nor] | White solid | 121.8 | 65.1 |
| [C3OMIM][Nor] | White viscous liquid | - | 44.1 |
| [C16Py][Nor] | Yellow viscous liquid | - | 5.9 |
| Compounds | K. pneumoniae | RDIC50 | S. aureus | RDIC50 | B. subtilis | RDIC50 |
|---|---|---|---|---|---|---|
| Cip | 196.50 | - | 29.16 | - | 3.84 | - |
| [Cip][Mes] | 55.07 | 3.6 | 31.96 | 0.91 | 14.38 | 0.3 |
| [Cip][Glu] | 74.52 | 2.6 | 29.81 | 1.0 | 12.16 | 0.3 |
| [Cip][Gly] | 82.79 | 2.4 | 24.06 | 1.2 | 19.78 | 0.2 |
| [Ch][Cip] | 51.12 | 3.8 | 181.40 | 0.2 | 20.60 | 0.2 |
| [EMIM][Cip] | 64.80 | 3.0 | 24.24 | 1.2 | 16.17 | 0.2 |
| [C2OHMIM][Cip] | 36.42 | 5.4 | 61.95 | 0.5 | 15.75 | 0.2 |
| [C2OHDMIM][Cip] | 50.99 | 3.9 | 21.20 | 1.4 | 12.35 | 0.3 |
| [C3OMIM][Cip] | 47.61 | 4.1 | 82.05 | 0.4 | 21.66 | 0.2 |
| [C16Py][Cip] | 9.88 | 19.9 | 1430 | 0.0 | 6.07 | 0.6 |
| Nor | 255.2 | - | 123.4 | 117.2 | ||
| [Nor][Mes] | 203.8 | 1.3 | 230.3 | 0.5 | 65.12 | 1.8 |
| [Nor][Glu] | 225.2 | 1.1 | 176 | 0.7 | 98.67 | 1.2 |
| [Nor][Gly] | 242.4 | 1.1 | 118.2 | 1.0 | 67.49 | 1.7 |
| [Ch][Nor] | 199.4 | 1.3 | 127.1 | 1.0 | 95.49 | 1.2 |
| [EMIM][Nor] | 207.5 | 1.2 | 211.2 | 0.6 | 24.48 | 4.8 |
| [C2OHMIM][Nor] | 201.0 | 1.3 | 91.70 | 1.3 | 88.61 | 1.3 |
| [C2OHDMIM][Nor] | 178.2 | 1.4 | 79.55 | 1.6 | 148.8 | 0.8 |
| [C3OMIM][Nor] | 224.5 | 1.1 | 106.7 | 1.1 | 68.12 | 1.7 |
| [C16Py][Nor] | 202.9 | 1.3 | 10.84 | 11.4 | 87.29 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madeira, D.; Alves, C.; Silva, J.; Florindo, C.; Costa, A.; Petrovski, Ž.; Marrucho, I.M.; Pedrosa, R.; Santos, M.M.; Branco, L.C. Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials. Proceedings 2021, 78, 3. https://doi.org/10.3390/IECP2020-08649
Madeira D, Alves C, Silva J, Florindo C, Costa A, Petrovski Ž, Marrucho IM, Pedrosa R, Santos MM, Branco LC. Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials. Proceedings. 2021; 78(1):3. https://doi.org/10.3390/IECP2020-08649
Chicago/Turabian StyleMadeira, Diogo, Celso Alves, Joana Silva, Catarina Florindo, Alexandra Costa, Željko Petrovski, Isabel M. Marrucho, Rui Pedrosa, Miguel M. Santos, and Luís C. Branco. 2021. "Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials" Proceedings 78, no. 1: 3. https://doi.org/10.3390/IECP2020-08649
APA StyleMadeira, D., Alves, C., Silva, J., Florindo, C., Costa, A., Petrovski, Ž., Marrucho, I. M., Pedrosa, R., Santos, M. M., & Branco, L. C. (2021). Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials. Proceedings, 78(1), 3. https://doi.org/10.3390/IECP2020-08649










