1. Introduction
The release profile of a topical semisolid dosage form, extracted from in vitro release testing (IVRT), typically carried out through Franz diffusion cells, enables the determination of the in vitro release rate (IVRR). This kinetic parameter provides important information on the microstructure characteristics of the product, such as particle size and rheological behavior. For this reason, it is considered a product critical quality attribute [
1,
2,
3,
4,
5,
6]. In this context, the determination of the in vitro release profile is a valuable tool during product development and optimization [
7].
The present work aimed at providing the assumptions to assist a sustainable improvement of the pharmaceutical performance of an anti-inflammatory semisolid dosage form. The qualitative/quantitative composition and the production process were already well-established; however, there were some parameters that lacked optimization, since in pre-stability studies, a marked decrease on drug release outcomes was observed. To address this constraint, the historical data of the product were thoughtfully analyzed following Quality by Design (QbD) principles. This is referred to as “retrospective QbD” (rQbD), since it focuses on product historical data and not on classic QbD approaches, which are mainly directed towards new product development [
8].
A cause-and-effect diagram and a risk estimation matrix were constructed to identify potential CPPs (Critical Process Parameters) and CMAs (Critical Material Attributes) that could impact the formulation CQAs (Critical Quality Attributes). From this analysis (data not shown), three main factors were identified as critical: (i) mixing time; (ii) sodium hydroxide content and, finally, (iii) carbopol viscosity. Efforts were then made to rationalize, predict and ultimately maximize the effects of these parameters on the product pharmaceutical quality. For that, a 23 full factorial design was employed to assess the impact of the above mentioned variables on the pH, viscosity, IVRR and cumulative amount released at the end of the IVRT study. During this optimization phase, all manufactured batches were produced at a laboratory scale. To confirm these assumptions, the formulations were then translated from lab to industrial scale, envisioning the validation of the working conditions in line with the predefined quality target product profile (QTPP).
2. Materials and Methods
2.1. Materials
All formulation components (medium chain triglycerides, hydroxiethylcellulose, carbopol 980, propylene glycol, propylparabene, methylparabene, sodium diclofenac and sodium hydroxide) were kindly provided by Laboratórios Basi Indústria Farmacêutica S.A. (Mortágua, Portugal). The commercial name of the raw materials is not disclosed for confidential purposes. For IVRT tests, propylene glycol was acquired from Merck and phosphate buffered saline was purchased from Sigma. Water was purified (Millipore®) and filtered through a 0.22 µm nylon filter before use. All other chemicals were of analytical grade or equivalent.
2.2. Methods
2.2.1. Diclofenac Emulgel Production
Emulgels regard pharmaceutical dosage forms gathering emulsion and gel properties, which enables their use as a controlled topical delivery system [
9]. Their production firstly involves the preparation of an emulsion by the hot emulsification method. Briefly, water, medium chain triglycerides, hydroxiethylcellulose and the carbopol were mixed with propylene glycol, propylparabene and methylparabene, which had been previously heated to 40 °C to enable the complete solubilization of both preservatives. Note that different carbopol viscosities were used, as this was one of the critical material attributes (CMAs) retrieved from the risk assessment analysis. Both phases were homogenized by an Ultra-Turrax (T50B IKA) for a specified rotation, time and temperature. Afterwards, the drug was dissolved in water at 70 °C and blended into the previously prepared mixture by using a mechanical stirrer (Heidolph AZA 2051). The formulation was then cooled down to 25 °C and a 10%(
w/V) sodium hydroxide solution was slowly added following fixed intervals of time according to the design of experiments (DoE). The formulation was then filled into suitable lined collapsible aluminum tubes (100 g). Then, 1 kg batches were considered for laboratory scale studies, whilst 600 kg batches were considered for industrial scale.
2.2.2. Quality Target Product Profile (QTPP) Definition
The establishment of a QTPP is regarded as the basis of formulation development, as it refers to a prospective summary of the quality characteristics intended for the product [
10]. Therefore, the QTPP was established envisioning the emulgel quality features intended to reach, considering the drug product efficacy and safety aspects.
2.2.3. Retrospective Quality by Design Applied to Diclofenac Formulation Optimization
Since the qualitative/quantitative composition and the production process were already well-disclosed, it was possible, based on prior knowledge, to retrospectively identify several production settings/critical material attributes which might have a direct repercussion on the formulation. The (i) neutralizer addition (final hydroxide concentration) and (ii) thickener grade (carbopol viscosity) were considered as CMAs. On the other hand, as CPPs, the mixing time (40 vs. 80 min) during production was selected. As critical quality attributes (CQAs), due to their overall importance in semisolid microstructure, the following parameters were considered: viscosity, pH and IVRT outputs—IVRR and cumulative amount of drug released at the end of the study (Qf).
A 2
3 full factorial design was performed for the optimization of the diclofenac emulgel formulation. This design envisions an in-depth analysis of the impact and interactions between the previously referred CMAs and CPP, in the formulation CQAs. Coded (−1, +1) levels were used for each independent variable, X
1, X
2 and X
3 (mixing time, NaOH final product concentration and carbopol viscosity), in which the −1 level corresponds to the lower value of each variable and +1 to the upper one.
Table 1 describes the settings used for each formulation. The experimental design and the polynomial models were solved resorting to JMP Pro software. These models were used to describe the influence of each factor and to check for potential synergisms between them.
Equation (1) defines the polynomial equation used to describe the behavior of each selected independent variable.
where Y refers to the response in the absence of effects; β
1, β
2 and β
3 the linear coefficients of the independent variables; and β
12, β
13 and β
23 the interaction coefficients between the factors. Analysis of variance (ANOVA) and Student’s
t-test were applied to test pair-wise multiple comparisons. A value of
p < 0.05 was considered statistically significant.
2.2.4. pH Measurement
Topical products should be manufactured with an appropriate pH range in order to assure an adequate drug solubility, stability and ultimately product biocompatibility. Moreover, pH values were determined at room temperature (25 °C), in triplicate, using a digital pH meter pH/ION seven compact—Metler Toledo, previously calibrated using standard buffer solutions (pH of 4.00, 7.00 and 10.00). About 1 g of each sample was weighed and dispersed in 10 times the volume of distilled water. Afterwards, the respective pH value was recorded. The determination was performed 24 h after batch manufacturing.
2.2.5. Viscosity Measurement
Formulation viscosity was evaluated 24 h after production at 25 °C, using a rotational viscometer (Brookfield Viscosimeter®, RV DV-II, Brookfield engineering laboratories, Inc., Middleboro, MA, USA) with a spindle T-A.
2.2.6. In Vitro Release Testing and HPLC Analysis
The IVRT method was conducted using static vertical Franz diffusion cells (PermeGear, Inc., Hellertown, PA, USA) with a diffusion area of 0.636 cm
2 and a receptor compartment of 5 mL. Then, 300 mg of the formulation was applied in the donor compartment, separated from the receptor compartment by a polysulfone membrane, previously soaked in distilled water for 30 min. The receptor media comprised a phosphate buffered saline (PBS): propylene glycol mixture (80:20,
V/V), continuously stirred at 600 rpm and maintained at a temperature of 37 °C. Samples of the receptor phase were withdrawn at 15, 30, 60, 90, 120, 150 and 180 min. After each collection, the same volume of medium was replaced with receptor solution. A n = 4 was performed in the same conditions. The concentration of diclofenac in IVRT samples was determined through HPLC, following the experimental procedures previously described [
7,
11].
3. Results
3.1. QTPP Definition
To follow a rQbD-based development approach, it is essential to define the desired product performance profile, also known as quality target product profile (QTPP). This refers to a prospective summary of quality characteristics to be achieved for a pharmaceutical product [
12]. Taking into account the defined QTPP, presented in
Table 2, as well as the historical data gathered during the initial development studies, it was possible to identify CPP, CMA and CQA pertaining to the diclofenac emulgel formulation. This information was then integrated within quality risk management principles and with DoE, to effectively apply QbD principles [
8].
CQA, Critical Quality Attribute; IVRR, in vitro release rate; Qf, cumulative amount of drug released in the end of the study; rQbD, retrospective Quality by Design.
3.2. Quality by Design Outputs
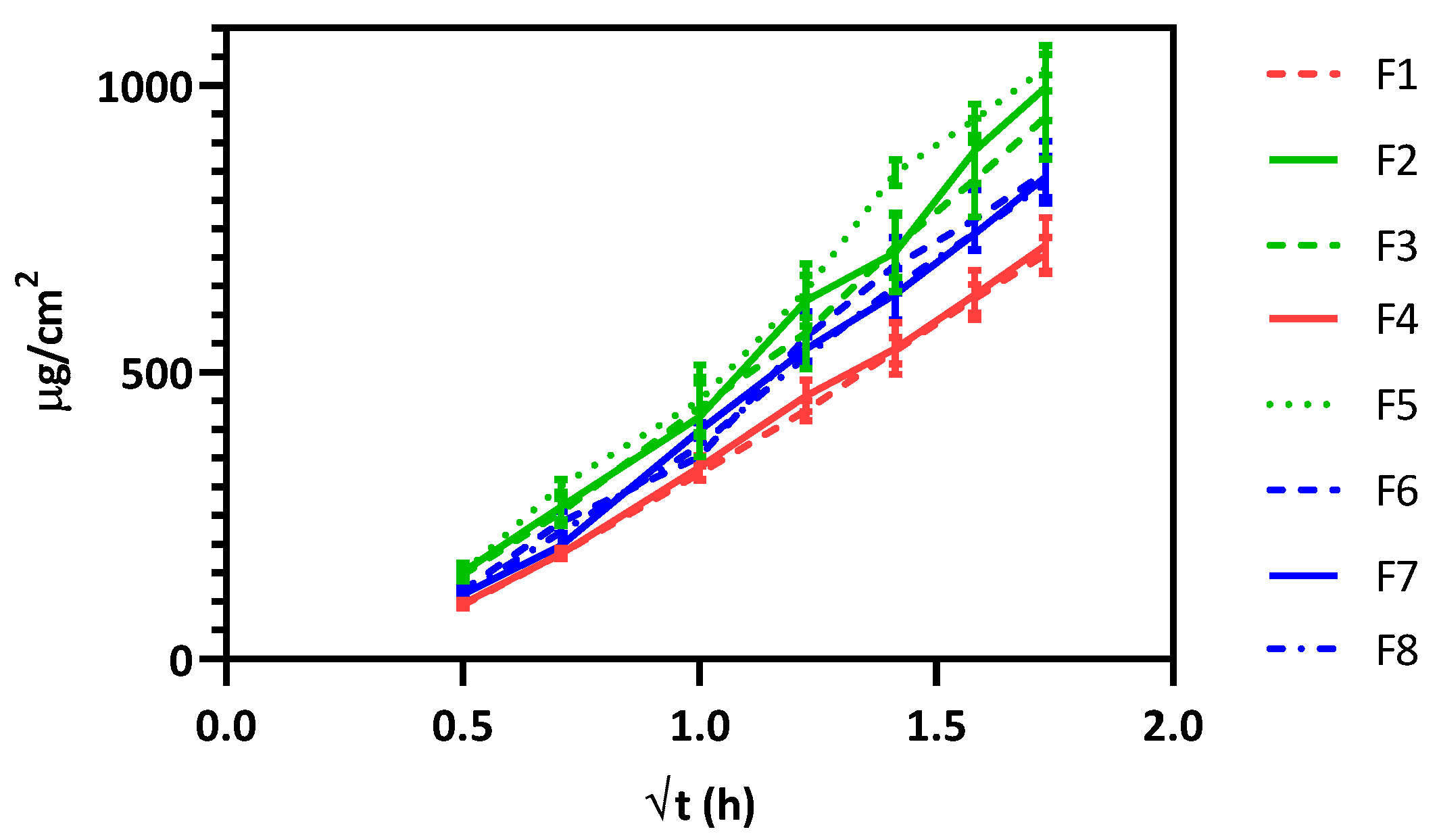
As presented in
Figure 1, there are distinct release behaviors among the formulations. Three principal groups can be observed: (i) high release (F2, F3, and F5), (ii) moderate release (F6, F7 and F8) and (iii) low release (F4, and F1).
Table 3 and
Table 4 gather the values of the coefficients obtained from the experimental design, as well as the corresponding statistical significance.
4. Discussion
A higher positive coefficient indicates that an increase of that specific CMA/CPP promotes an increase in the response, whilst a negative coefficient bears the opposite system response, meaning that, with its increase, the system response decreases. The higher the magnitude of the coefficients, the higher is the influence of that variable on the system, either positively or negatively.
As can be seen from
Table 2, higher coefficient magnitudes are attained for sodium hydroxide content (β
2) and carbopol viscosity (β
3). These are the main CMA that affect formulation CQA, meaning that formulation parameters prevail over the CPP. The sodium hydroxide content negatively affects the final product viscosity, resulting in a better product performance in terms of release behavior. Similarly, carbopol viscosity also negatively impacts formulation viscosity, i.e., higher carbopol viscosity values prompt a decrease in final product viscosity, which, in turn, is correlated with higher IVRT outputs. Another important analysis relies on the interaction terms, which indicate how the variation of one factor may modulate the response of another one, and consequently influence the selected response. Regarding possible synergistic effects between the studied CMA/CPP, only the coefficient β
13 registered higher magnitudes.
It should be remarked that, despite the pH had been selected as a product CQA, this individual parameter showed no significant effect (p-value > 0.05, data not shown).
As topical semisolid microstructure is highly dependent on batch size, these assumptions needed to be further validated at an industrial scale. In order to draw plausible conclusions two opposite formulations were produced: one with lower sodium hydroxide content and higher carbopol viscosity, and a second one with superior percentage of sodium hydroxide and lower carbopol viscosity. The results, presented in
Figure 2, sustain DoE estimates.
5. Conclusions
Based on the DoE results, the following assumptions may be retrieved: (i) The principal effect seems to be correlated with the sodium hydroxide concentration. In other words, to yield a superior formulation performance, the concentration of hydroxide should be set to the higher level. This will enable a decrease in final product viscosity, which, in turn, promotes a superior IVRT performance. (ii) A secondary effect regards carbopol viscosity, with lower values yielding lower IVRT outputs. These results were further confirmed at an industrial scale.
Such findings consubstantiate the relevance of the application of rQbD principles in the redesign of pharmaceutical drug products.
Author Contributions
Conceptualization, C.C. and C.V. (Carla Vitorino); methodology, M.M. and C.V. (Cláudia Veloso); software, M.M.; formal analysis, M.M.; investigation, M.M. and C.V. (Cláudia Veloso); resources, C.V. (Carla Vitorino) and C.C.; data curation, M.M.; writing—original draft preparation, M.M.; writing, review and editing, M.M.; visualization, C.V. (Carla Vitorino); supervision, C.C. and C.V. (Carla Vitorino); funding acquisition, C.C. and C.V. (Carla Vitorino). All authors have read and agreed to the published version of the manuscript.
Funding
Margarida Miranda and Claudia Veloso acknowledge the PhD grants PD/BDE/135075/2017 and PD/BDE/150302/2019, respectively, assigned by FCT and Laboratórios Basi from Drugs R&D Doctoral Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors also acknowledge Coimbra Chemistry Center (CQC), supported by FCT, through the project UID/QUI/00313/2020. The authors acknowledge Laboratórios Basi–Indústria Farmacêutica S.A., specially to Ricardo Dias, Catarina Marques, Alvaro Santos and Sónia Santos for assisting with the diclofenac emulgel formulations production.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CMA | critical material attributes; |
| CPP | critical process parameters; |
| CQA | critical quality attributes; |
| DoE | design of experiments; |
| IVRR | in vitro release rate; |
| IVRT | in vitro release testing; |
| QbD | Quality by Design; |
| Qf | Cumulative amount of drug released in the end of the IVRT study; |
| QTPP | quality target product profile. |
References
- FDA. Guidance for Industry: Nonsterile Semisolid Dosage Forms: Scale-Up and Postapproval Changes: Chemistry, Manufacturing, and Controls: In Vitro Release Testing and In Vivo Bioequivalence Documentation; FDA: Rockville, MD, USA, 1997. [Google Scholar]
- Braddy, A.C.; Davit, B.M.; Stier, E.M.; Conner, D.P. Survey of International Regulatory Bioequivalence Recommendations for Approval of Generic Topical Dermatological Drug Products. AAPS J. 2015, 17, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.L.; Shah, V.P.; Tenjarla, S.N.; Corbo, M.; DeMagistris, D.; Feldman, T.G.; Franz, T.J.; Miran, D.R.; Pearce, D.M.; Sequeira, J.A. Assessment of value and applications of in vitro testing of topical dermatological drug products. Pharm. Res. 1999, 16, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidance Notes on Dermal Absorption Draft 22 October 2010; OECD: Paris, France, 2010; pp. 1–53. [Google Scholar]
- Sivaraman, A.; Banga, A. Quality by design approaches for topical dermatological dosage forms. Res. Rep. Transdermal Drug Deliv. 2015, 4, 9–21. [Google Scholar] [CrossRef]
- Dandamudi, S. In Vitro Bioequivalence Data for a Topical Product. In Proceedings of the FDA Workshop on Bioequivalence Testing of Topical Drug Products, Silver Spring, MD, USA, 20 October 2017. [Google Scholar]
- Miranda, M.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. aQbD as a platform for IVRT method development–A regulatory oriented approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.M.A.; Vicente, S.; Cunha, S.; Coelho, J.F.J.; Silva, C.; Reis, M.S.; Simões, S. Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films. Int. J. Pharm. 2017, 528, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.D.; Sharma, H.; Jeswani, G.; Jha, A.K. Novel Gels: Implications; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- ICH Pharmaceutical Development Q8. ICH Harmon. Tripart. Guidel. 2009, 8, 1–28.
- Miranda, M.; Cova, T.; Augusto, C.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. Diving into Batch-to-Batch Variability of Topical Products-a Regulatory Bottleneck. Pharm. Res. 2020, 37, 218. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Miranda, A.; Cova, T.; Gonçalves, L.; Almeida, A.J.; Sousa, J.J.; do Vale, M.L.C.; Marques, E.F.; Pais, A.; Vitorino, C. Modeling of ultra-small lipid nanoparticle surface charge for targeting glioblastoma. Eur. J. Pharm. Sci. 2018, 117, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, C.; Liu, J.; Fang, L. Correlation between rheological properties, in vitro release, and percutaneous permeation of tetrahydropalmatine. AAPS PharmSciTech 2011, 12, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; Veiga, F.; Vitorino, C. Progressing Towards the Sustainable Development of Cream Formulations. Pharmaceutics 2020, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; Miranda, M.; Cardoso, C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Alves, L.; Antunes, F.E.; Sousa, J.J.; Pais, A.A.C.C. Design of a dual nanostructured lipid carrier formulation based on physicochemical, rheological, and mechanical properties. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).