Abstract
The intranasal administration of nanostructured lipid carriers (NLCs) has been suggested as a promising strategy to improve the fast treatment of epilepsy. This route allows for drug passage directly from the nose to the brain, avoiding the need of bypassing the blood–brain barrier. In addition, the quality-by-design (QbD) approach is a useful tool for the optimization of manufacturing variables, resulting in effective and safe pharmaceutical formulations. The aim of this work was to use the QbD approach to optimize a NLCs formulation for the nose-to-brain delivery of diazepam. The studies began with the screening of excipients and the assessment of the lipid-drug compatibility. The central composite design was used to evaluate the effects of critical material attributes (CMAs) (ratio of solid and liquid lipids and the amount of drug and emulsifiers) on the CQAs of the diazepam-loaded NLCs formulation (particle size, polydispersity index (PDI), zeta potential (ZP) and encapsulation efficiency (EE)). The results showed that the most adequate ratios of lipids and emulsifiers were 6.65:2.85 and 4.2:0.3 (%, w/w), with values of 84.92 nm, 0.18, −18.20 mV and 95.48% for particle size, PDI, ZP and EE, respectively. This formulation was selected for further studies related to the optimization of critical process parameters (CPPs).
1. Introduction
Neurological disorders, including epilepsy, require a rapid and effective treatment targeting the brain. In this area, the intranasal administration of lipid nanosystems, such as nanostructured lipid carriers (NLCs) has been suggested as a promising strategy. This route allows for drug passage directly from the nose to the brain, avoiding the need of bypassing the blood–brain barrier [1]. The quality-by-design (QbD) approach has been applied to optimize NLCs formulations, improving the manufacturing processes and ensuring the quality and safety of the final products. Herein, the quality target product profile (QTPP) and critical quality attributes (CQAs) are identified, and a risk assessment analysis is conducted to evaluate the critical material attributes (CMAs) and critical process parameters (CPPs) [2]. The aim of this work was to use the QbD approach to optimize a NLCs formulation for the nose-to-brain delivery of diazepam, improving epilepsy emergency treatment. Studies started with the screening of excipients and evaluation of the lipid-drug compatibility. Subsequently, the QbD approach was applied to evaluate the effects of CMAs on CQAs in the diazepam-loaded NLCs formulation.
2. Experiments
2.1. Materials
Diazepam was purchased from Acofarma (Barcelona, Spain). Precirol® 5 ATO (glyceryl palmitostearate), Compritol® 888 ATO (glyceryl behenate), Gelucire® 43/01 (hard fat compounds), Gelucire® 44/14 (lauroyl polyoxyl-32 glycerides),Gelucire® 50/13 (stearoyl polyoxyl-32 glycerides), cetyl palmitate, Apifil® (PEG-8 beeswax), Labrafac® W1349 (medium chain triglycerides) and Capryol® 90 (propylene glycol monocaprylate) were kindly provided by Gattefossé (Lyon, France). Imwitor® 900K (glyceryl stearate), Softisan® 100 (hydrogenated coco-glycerides), Softisan® 154 (hydrogenated palm oil), Dynasan® 118 (glyceryl tristearate) and Witepsol® E85 (hard fat compounds) were from Oxi-med (Barcelona, Spain). Cetiol® V (decyl oleate) and glyceryl monostearate were from Guinama (Valencia, Spain). Miglyol® 812 (medium-chain triglycerides of caprylic and capric acids), Tween 80® (polysorbate 80), sodium deoxycholate, oleic acid, isopropyl myristate, vitamin E, chloride benzalkonium, stearic acid, sodium chloride and sodium phosphate were purchased from Acofarma (Barcelona, Spain). Lutrol® F68 (poloxamer 188) and Lutrol® F127 (poloxamer 407) were acquired from BASF (Gordon, USA), Phospolipon® 90 G and Phospolipon® 90 H were obtained from Lipoid (Ludwigshafen am Rhein, Germany) and acetonitrile was from Thermo Fisher Scientific (Loughborough, UK). The purified water used for the NLCs production was obtained from a Direct-Q® Ultrapure Water Systems, Merck Millipore (Darmstadt, Germany).
2.2. Methods
2.2.1. Screening of Excipients
The excipients chosen to develop the NLCs were based on previous research, where the suitability of lipids and emulsifiers for nasal administration was confirmed [3,4]. Tested solid lipids were Precirol® 5 ATO, Imwitor® 900K, Compritol® 888 ATO, Gelucire® 43/01, Gelucire® 44/14, Gelucire® 50/13, glyceryl monostearate, stearic acid, cetyl palmitate, Softisan® 100, Softisan® 154, Dynasan® 118, Apifil® and Witepsol® E85. Tested liquid lipids were Miglyol® 812, oleic acid, isopropyl myristate, Cetiol® V, vitamin E, Labrafac® W1349, Capryol® 90 and Microcare®. The tested emulsifiers were non-ionic emulsifiers, such as Tween 80®, Lutrol® F68, Lutrol® F127, and anionic emulsifiers, such as sodium deoxycholate and phospholipids (Phospolipon® 90 G and Phospolipon® 90 H).
2.2.2. Compatibility between Solid and Liquid Lipids
Compatibility between lipids was evaluated by screening different ratios of solid and liquid lipids, i.e., 60:40, 70:30, 80:20 and 90:10, heated 5–10 °C above the melting point of the solid lipid under stirring for 1 h, and cooled at room temperature (25 ± 0.5 °C). The mixture was examined for any phase separation and color change. Afterwards, the mixture was placed in a hydrophilic filter paper, followed by visual observation to determine the presence/absence of liquid oil droplets on the filter to detect the existence/absence of immiscibility.
2.2.3. Drug-Lipid Solubility
To evaluate which solid lipid solubilizes the highest diazepam amount, an excess of the drug (5–10%, w/w) was added to the lipid and heated to 5–10 °C above the melting point under continuous stirring for 1 h. After solidification by cooling to room temperature, the presence/absence of insoluble drug crystals was observed. The same procedure was conducted with the liquid lipid.
2.2.4. Preparation of the Diazepam-Loaded NLCs Formulation
Diazepam-loaded NLCs were prepared from the method previously employed by Silva et al. [5]. Briefly, the aqueous and lipid phases were heated to 5–10 °C above the melting point of the solid lipid. Afterwards, the aqueous phase was added to the lipid phase and the mixture was emulsified under high-speed stirring using an Ultra-Turrax® T25 (Janke and Kunkel GmbH (Staufen, Germany) at 13,400 rpm for 5 min. The formed emulsion was sonicated, with an amplitude of 75% for 15 min, using a VCX 130 ultrasonic processor (Sonics, Switzerland). The obtained O/W nano-emulsion was transferred to glass vials and drastically cooled down to room temperature (20 ± 0.5 °C) to generate NLCs.
2.2.5. Characterization of the Diazepam-Loaded NLCs Formulation
Particle size was evaluated by laser diffractometry (Mastersizer 3000, Malvern, UK) and dynamic light scattering (DLS), using a Malvern Nano-Zetasizer (Malvern, UK). The polydispersity index (PDI) and zeta potential (ZP) were evaluated using the same Malvern Nano-Zetasizer.
The encapsulation efficiency (EE) of diazepam in the NLCs was calculated according to the following equation: EE (%) = [(total amount of drug − free drug)/total amount of drug] × 100.
Diazepam was quantified by high-pressure liquid homogenization (HPLC), at 254 nm.
2.2.6. QbD Approach
The QbD approach was applied to optimize the diazepam-loaded NLCs formulation, improving the manufacturing process and ensuring the quality and safety of the final product. The effect of the CMAs and CPPs on the CQAs is shown in Figure 1.
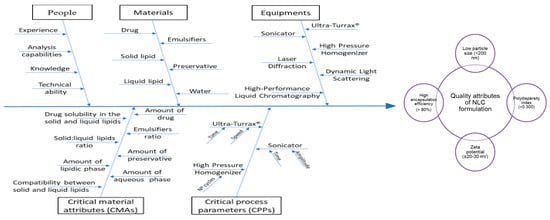
Figure 1.
Ishikawa diagram showing the effects of critical material attributes (CMAs) and critical process parameters (CPPs) on the critical quality attributes (CQAs), for the optimization of a nanostructured lipid carrier (NLCs) formulation.
3. Results
3.1. Preparation and Characterization of the Diazepam-Loaded NLCs Formulation
Precirol® 5 ATO and Cetiol® V were selected as the solid lipid (SL) and the liquid lipid (LL), respectively. Tween 80® and sodium deoxycholate were selected as emulsifiers and a drug concentration of 0.50% was selected to prepare the diazepam-loaded NLCs formulation (Table 1).

Table 1.
Composition of diazepam-loaded nanostructured lipid carriers (NLCs) formulation.
3.2. QbD Approach
Central Composite Design (CCD)
CCD was used to evaluate the effects of CMAs on the CQAs of the NLCs formulation (Table 2 and Table 3 and Figure 2).

Table 2.
Selection of the central composite design (CCD) variables and respective levels.

Table 3.
Effect of the critical material attributes (CMAs) on the critical quality attributes (CQAs) of the diazepam-loaded NLCs.
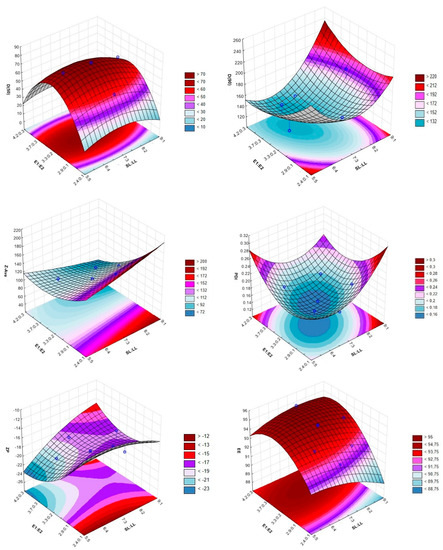
Figure 2.
The 3D surface plots portraying the effect of the ratio between the solid and liquid lipids (SL:LL) and the two emulsifiers (E1:E2) on the size (D(50): 50% of particles with size equal to or lower than the given value, D(90): 90% of particles with size equal to or lower than the given value and Z-Ave: mean particle size), polydispersity index (PDI), zeta potential (ZP) and encapsulation efficiency (EE).
4. Discussion
The studies for screening excipients, compatibility between lipids and the drug-lipid solubility allowed for the preparation of a diazepam-loaded NLCs formulation with the typical characteristics of these systems, such as low viscosity and milky appearance. In addition, the use of the QbD approach was effective for the optimization of the diazepam-loaded NLCs formulation. Other studies have also reported the effectiveness of QbD optimizing NLCs formulations. For example, Cunha et al. used the QbD and two different experiment designs (CCD and Box-Behnken design) to optimize a rivastigmine-loaded NLCs formulation prepared through two different production methods (sonication and high-pressure homogenization). In this study, the variations in the CMAs and CPPs originated important outcomes in the CQAs of the final formulation [6]. From Table 3 and Figure 2, it can be observed that the most adequate ratios of lipids and emulsifiers were 6.65:2.85 and 4.2:0.3 (%, w/w). The results of particle size, PDI, ZP and EE were, respectively, 84.92 nm, 0.178, −18.20 mV and 95.48%. These values are in accordance with the requisites of intranasal delivery of NLCs formulations, which are a particle size of less than 200 nm, a PDI less than 0.3, a ZP close to −20 mV and an EE higher than 80% [6,7,8].
5. Conclusions
Optimizing NLCs formulations is critical to achieve a reproducible quality of the final pharmaceutical products, in terms of both efficacy and safety. The QbD approach is a useful tool for the development of these systems, being observed that manufacturing variables related to the materials and process parameters are important for the optimization of the diazepam-loaded NLCs formulation.
The diazepam-loaded NLCs formulation with the best CQAs was selected for further optimization, related to the selection of the best CPPs and using the same design of experiment, which will be tested in vitro and in vivo in the future.
Author Contributions
C.P.C. and A.C.S. conceived and designed the experiments and methodology; C.P.C. performed the experiments; C.P.C., S.C., A.F.P. and A.C.S. analyzed the data; C.P.C. wrote the original draft preparation; A.C.S., J.N.M. and J.M.S.L. revised the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (FCT), Portugal (SFRH/BD/136177/2018) and by the Applied Molecular Biosciences Unit-UCIBIO, which is financed by national funds from FCT/MCTES (UIDMulti/04378/2019).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CCD | central composite design |
| CMAs | critical material attributes |
| CPPs | critical process parameters |
| CQAs | critical quality attributes |
| DLS | dynamic light scattering |
| E1 | Tween®80 |
| E2 | sodium deoxycholate |
| EE | encapsulation efficiency |
| HPLC | high-pressure liquid homogenization |
| LL | liquid lipid |
| NLCs | nanostructured lipid carriers |
| PDI | polydispersity index |
| QbD | quality-by-design |
| QTPP | quality target profile product |
| SL | solid lipid |
| ZP | zeta potential |
References
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Costa, C.; Moreira, J.; Lobo, J.; Silva, A. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. Nanomedicine 2020, 28, 102206. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Lipid nanoparticles for nasal/intranasal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; González-Mira, E.; García, M. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Lobo, J.M.S.; Silva, A.C. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Mahbubul, I. Introduction to Nanofluid. In Preparation, Characterization, Properties and Application of Nanofluid; Mahbubul, I.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 1–13. [Google Scholar]
- Mahbubul, I. Stability and Dispersion Characterization of Nanofluid. In Preparation, Characterization, Properties and Application of Nanofluid; Mahbubul, I.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 47–112. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).