Designing Ultra-Small Nanostructured Lipid Carriers: Critical Process Parameters †
Abstract
:1. Introduction
2. Experiments Materials
2.1. Optimization of the Production Method of NLC
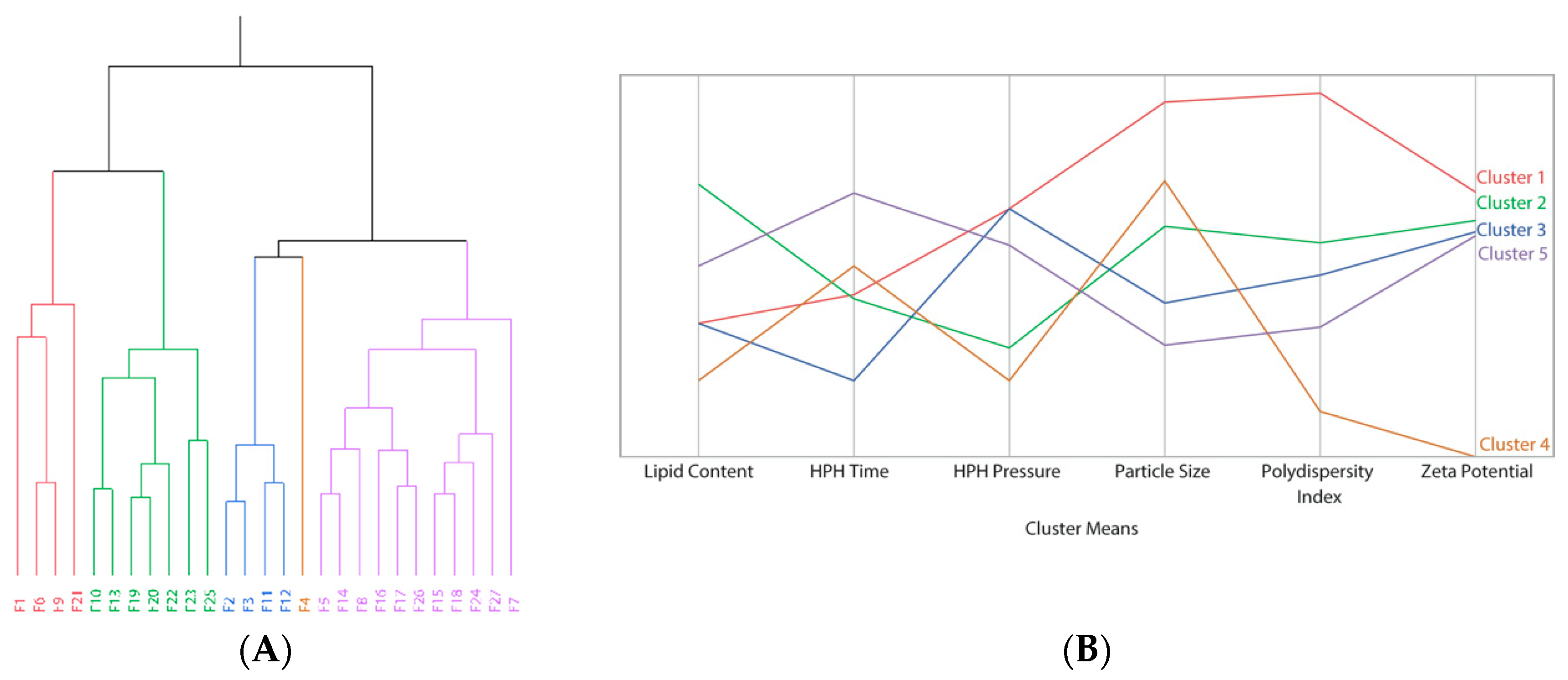
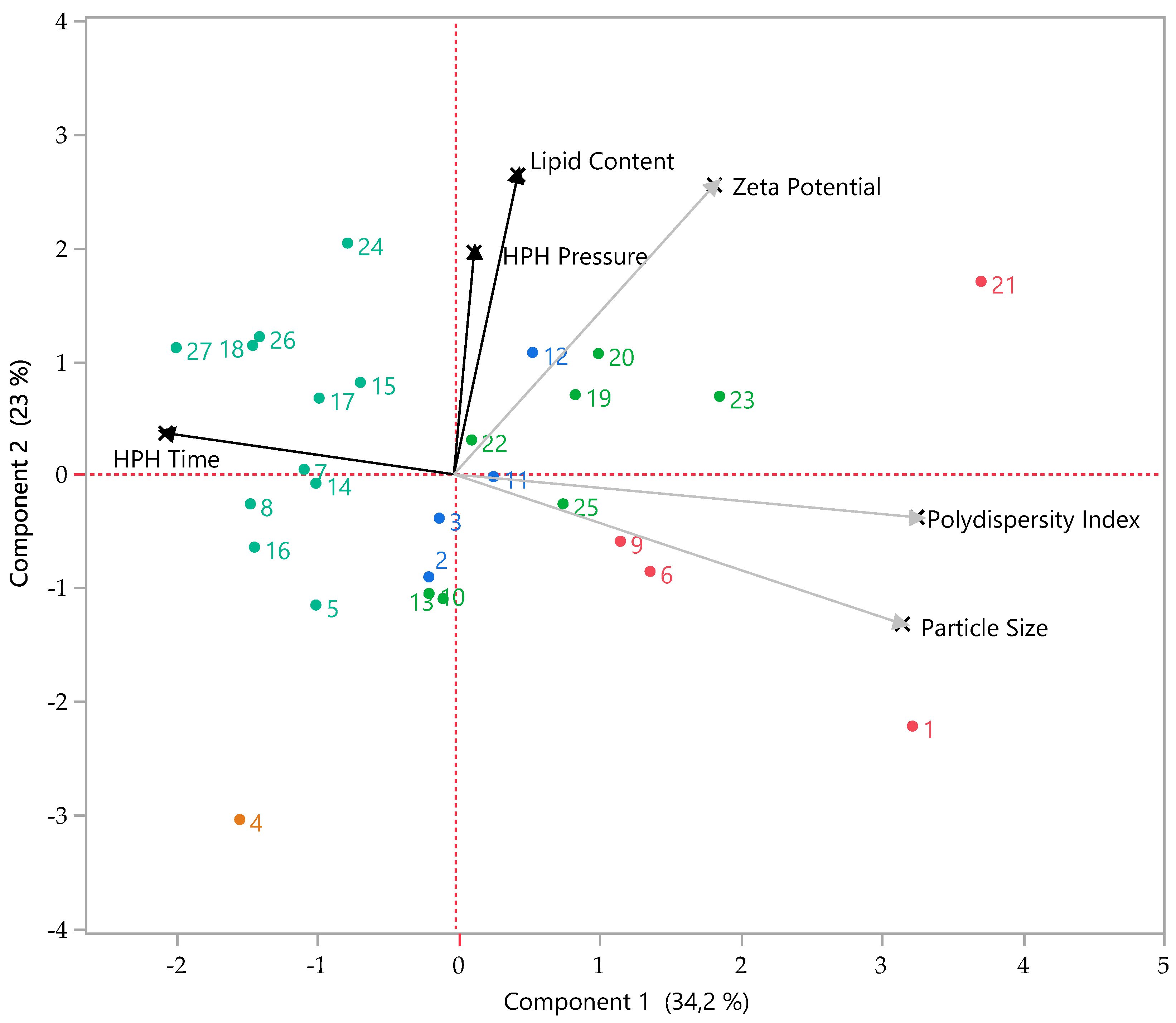
2.2. Multivariate Analyses
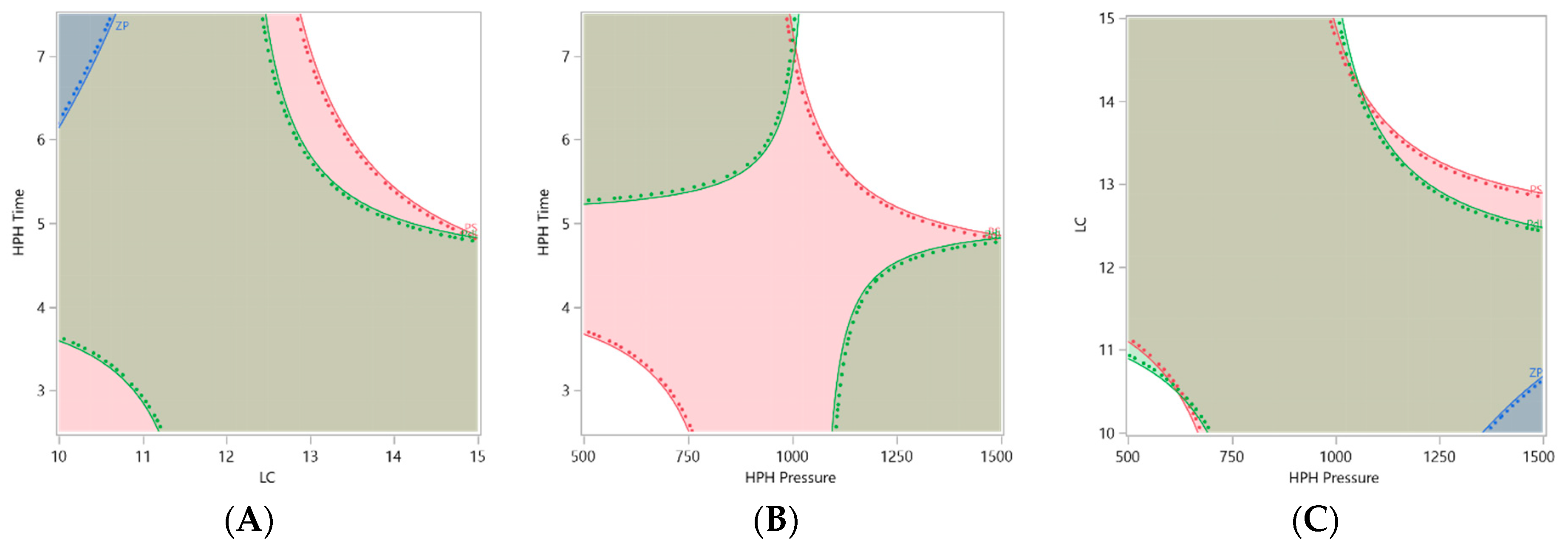
3. Results
Optimization and Production of Unloaded usNLCs
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| usNLC | Ultra-small nanostructured lipid carriers |
| NPs | Nanoparticles |
| QbD | Quality by design |
| HPH | High-pressure homogenization |
| CPP | Critical process parameters |
| CMAs | Critical material attributes |
| CQAs | Critical quality attributes |
| LC | Lipid content |
| PS | Particle size |
| PI | Polydispersity index |
| ZP | Zeta potential |
| F | Formulation |
References
- Patel, G.M.; Shelat, P.K.; Lalwani, A.N. QbD based development of proliposome of lopinavir for improved oral bioavailability. Eur. J. Pharm. Sci. 2017, 108, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Costa, C.P.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Using the quality by design (QbD) approach to optimize formulations of lipid nanoparticles and nanoemulsions: A review. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102206. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bio-active food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Monteagudo, S.M.; Yan, B.; Balasubramaniam, V.M. Engineering Process Characterization of High-Pressure Homogenization—From Laboratory to Industrial Scale. Food Eng. Rev. 2016, 9, 143–169. [Google Scholar] [CrossRef]
- Dingler, A.; Gohla, S. Production of solid lipid nanoparticles (SLN): scaling up feasibilities. J. Microencapsul. 2002, 19, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gupta SNRCN. Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Thassu, D.; Deleers, M.; Pathak, Y.V.; Pathak, Y. Nanoparticulate Drug Delivery Systems [Internet]. Taylor & Francis. (Drugs and the Pharmaceutical Sciences). 2007. Available online: https://books.google.pt/books?id=B-hsAAAAMAAJ (accessed on 30 November 2020).
- Barone, A.; Mendes, M.; Cabral, C.; Mare, R.; Paolino, D.; Vitorino, C. Hybrid Nanostructured Films for Topical Administration of Simvastatin as Coadjuvant Treatment of Melanoma. J. Pharm. Sci. 2019, 108, 3396–3407. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Soares, H.; Arnaut, L.; Sousa, J.; Pais, A.; Vitorino, C. Can lipid nanoparticles improve intestinal absorption? Int. J. Pharm. 2016, 515, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Basso, J.; Silva, J.; Cova, T.; Sousa, J.; Pais, A.; Vitorino, C. Biomimeting ultra-small lipid nanoconstructs for glioblastoma treatment: A computationally guided experimental approach. Int. J. Pharm. 2020, 587, 119661. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.; Mendes, M.; Silva, J.; Sereno, J.; Cova, T.; Oliveira, R.; Fortuna, A.; Castelo-Branco, M.; Falcão, A.; Sousa, J.; et al. Peptide-lipid nanoconstructs act site-specifically towards glioblastoma growth impairment. Eur. J. Pharm. Biopharm. 2020, 155, 177–189. [Google Scholar] [CrossRef] [PubMed]
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| Lipid content (% w/w) | 10 | 12.5 | 15 |
| High-pressure homogenization (HPH) time (min) | 2.5 | 5 | 7.5 |
| High-pressure homogenization (HPH) pressure (bar) | 500 | 1000 | 1500 |
| Dependent variables | Particle size (PS) | ||
| Polydispersity index (PI) | |||
| Zeta potential (ZP) | |||
| F | LC (%) | HPH Time (min) | HPH Pressure (bar) | PS | PI | ZP |
|---|---|---|---|---|---|---|
| 1 | 10 | 2.5 | 500 | 201 ± 0.4 | 0.414 | −34.3 ± 0.4 |
| 2 | 10 | 2.5 | 1000 | 112 ± 0.5 | 0.253 | −36.0 ± 0.5 |
| 3 | 10 | 2.5 | 1500 | 113 ± 0.4 | 0.254 | −36.0 ± 0.4 |
| 4 | 10 | 5 | 500 | 153 ± 1 | 0.161 | −43 ± 1 |
| 5 | 10 | 5 | 1000 | 110 ± 1 | 0.245 | −38 ± 1 |
| 6 | 10 | 5 | 1500 | 171 ± 2 | 0.333 | −35 ± 2 |
| 7 | 10 | 7.5 | 500 | 120 ± 1 | 0.160 | −29 ± 1 |
| 8 | 10 | 7.5 | 1000 | 106 ± 1 | 0.205 | −34 ± 1 |
| 9 | 10 | 7.5 | 1500 | 157 ± 1 | 0.386 | −35 ± 1 |
| 10 | 12.5 | 2.5 | 500 | 116 ± 2 | 0.256 | −38 ± 2 |
| 11 | 12.5 | 2.5 | 1000 | 119 ± 1 | 0.260 | −35 ± 1 |
| 12 | 12.5 | 2.5 | 1500 | 115.7 ± 0.4 | 0.260 | −32.0 ± 0.4 |
| 13 | 12.5 | 5 | 500 | 137 ± 1 | 0.266 | −37 ± 1 |
| 14 | 12.5 | 5 | 1000 | 97 ± 2 | 0.246 | −37 ± 2 |
| 15 | 12.5 | 5 | 1500 | 100 ± 1 | 0.247 | −35 ± 2 |
| 16 | 12.5 | 7.5 | 500 | 100.5 ± 0.6 | 0.251 | −37 ± 1 |
| 17 | 12.5 | 7.5 | 1000 | 98 ± 1 | 0.253 | −33 ± 1 |
| 18 | 12.5 | 7.5 | 1500 | 96 ± 2 | 0.212 | −34 ± 2 |
| 19 | 15 | 2.5 | 500 | 125 ± 1 | 0.264 | −32 ± 2 |
| 20 | 15 | 2.5 | 1000 | 138 ± 1 | 0.257 | −32 ± 1 |
| 21 | 15 | 2.5 | 1500 | 180.4 ± 0.3 | 0.406 | −28.0 ± 0.3 |
| 22 | 15 | 5 | 500 | 138 ± 2 | 0.230 | −34 ± 2 |
| 23 | 15 | 5 | 1000 | 170 ± 1 | 0.331 | −32 ± 1 |
| 24 | 15 | 5 | 1500 | 102 ± 1 | 0.192 | −32 ± 1 |
| 25 | 15 | 7.5 | 500 | 150 ± 1 | 0.352 | −36 ± 1 |
| 26 | 15 | 7.5 | 1000 | 100.1 ± 0.2 | 0.206 | −34.5 ± 0.2 |
| 27 | 15 | 7.5 | 1500 | 92 ± 2 | 0.203 | −38 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, M.; Basso, J.; Sousa, J.; Pais, A.; Vitorino, C. Designing Ultra-Small Nanostructured Lipid Carriers: Critical Process Parameters. Proceedings 2021, 78, 50. https://doi.org/10.3390/IECP2020-08691
Mendes M, Basso J, Sousa J, Pais A, Vitorino C. Designing Ultra-Small Nanostructured Lipid Carriers: Critical Process Parameters. Proceedings. 2021; 78(1):50. https://doi.org/10.3390/IECP2020-08691
Chicago/Turabian StyleMendes, Maria, João Basso, João Sousa, Alberto Pais, and Carla Vitorino. 2021. "Designing Ultra-Small Nanostructured Lipid Carriers: Critical Process Parameters" Proceedings 78, no. 1: 50. https://doi.org/10.3390/IECP2020-08691
APA StyleMendes, M., Basso, J., Sousa, J., Pais, A., & Vitorino, C. (2021). Designing Ultra-Small Nanostructured Lipid Carriers: Critical Process Parameters. Proceedings, 78(1), 50. https://doi.org/10.3390/IECP2020-08691







