Development of Anti-TNFR Antibody-Conjugated Nanoparticles †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. AuNPs Synthesis
2.2.2. AuNPs Functionalization
2.2.3. AuNPs Grafting
2.2.4. Characterization
3. Results
3.1. Optimization of Gold Nanoparticles Synthesis Protocol
3.1.1. Controlling the Size and Concentration of Gold Nanoparticles
3.1.2. Functionalization of the Gold Nanoparticles
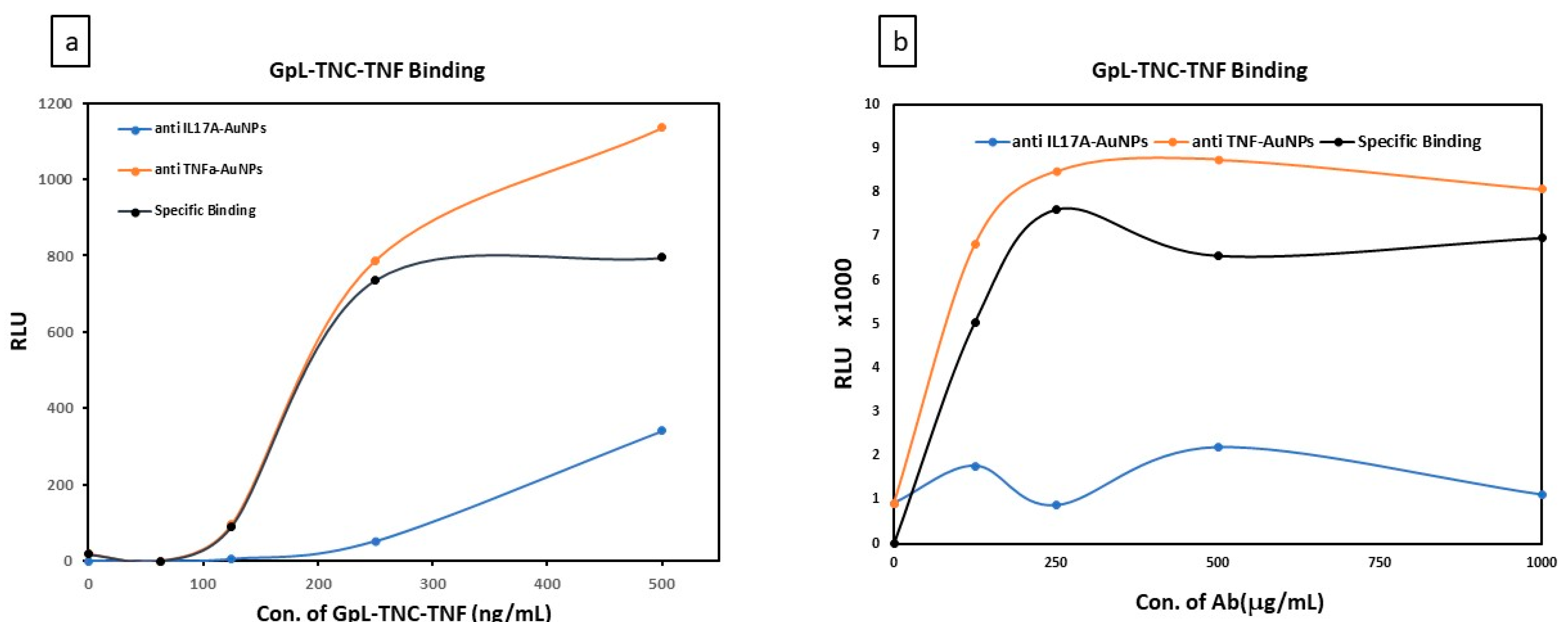
3.2. Characterization the Conjugation of C-AuNPs with Different Thersputics Antibodies
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abs | Antibodies |
| AuNPs | Gold nanoparticles |
| C-AuNPs | Carboxyl-modified gold nanoparticles |
| DLS | Dynamic light scattering |
| GpL | Gaussia princeps luciferase |
| IgG | Immunoglobulin G |
| PEG | Poly-ethylene glycol |
| PDL192 | Anti Fn14 monoclonal IgG1 antibody |
| SPR | Surface Plasmon Resonance |
| TNF | Tumor Necrosis Factor |
| TWEAK | TNF-related weak inducer of apoptosis |
References
- Bodmer, J.-L.; Schneider, P.; Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 2002, 27, 19–26. [Google Scholar] [CrossRef]
- Kelley, S.K.; Harris, L.A.; Xie, D.; Deforge, L.; Totpal, K.; Bussiere, J.; Fox, J.A. Preclinical Studies to Predict the Disposition of Apo2L/Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Humans: Characterization of in Vivo Efficacy, Pharmacokinetics, and Safety. J. Pharmacol. Exp. Ther. 2001, 299, 31–38. [Google Scholar] [PubMed]
- Alerts, E. Cachectin / tumor necrosis factor : production, distribution, and metabolic fate in vivo. J. Immunol. 2019, 135, 3972–3977. [Google Scholar]
- Medler, J.; Nelke, J.; Weisenberger, D.; Steinfatt, T.; Rothaug, M.; Berr, S.; Hünig, T.; Beilhack, A.; Wajant, H. TNFRSF receptor-specific antibody fusion proteins with targeting controlled FcγR-independent agonistic activity. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef]
- Wajant, H. Principles of antibody-mediated TNF receptor activation. Cell Death Differ. 2015, 22, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, P.; Sun, Y.; Xing, Y.; Yang, Y.; Dong, Y.; Xu, L.; Yang, Z.; Liu, D. A new strategy improves assembly efficiency of DNA mono-modified gold nanoparticles. Chem. Commun. (Camb.) 2011, 47, 5774–5776. [Google Scholar] [CrossRef]
- El-Sayed, I.H.; Huang, X.; El-Sayed, M.A. Surface Plasmon Resonance Scattering and Absorption of Anti-EGFR Antibody Conjugated Gold Nanoparticles in Cancer Diagnostics: Applications in Oral Cancer. Nano Letters 2005. Available online: https://kopernio.com/viewer?doi=10.1021/nl050074e&token=WzExNDE5NzUsIjEwLjEwMjEvbmwwNTAwNzRlIl0.-akvz5cNi2_mB0A37P4eWnqNTSM (accessed on 20 December 2019). [CrossRef]
- You, C.-C.; Arvizo, R.R.; Rotello, V.M. Regulation of alpha-chymotrypsin activity on the surface of substrate-functionalized gold nanoparticles. Chem. Commun. (Camb.) 2006, 2905–2907. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Lang, I.; Fu, S.; Wyzgol, A.; Fick, A.; Trebing, J.; Arana, A.C.; Weisenberger, D.; Wajant, H. Binding Studies of TNF Receptor Superfamily (TNFRSF) Receptors on Intact Cells. J. Biol. Chem. 2016, 291, 5022–5037. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions|Kopernio. Available online: https://kopernio.com/viewer?doi=10.1038/physci241020a0&token=WzExNDE5NzUsIjEwLjEwMzgvcGh5c2NpMjQxMDIwYTAiXQ.es09awC7jdG9BahFkjNV3cCqjpQ (accessed on 16 December 2019).
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Lomant, A.J.; Fairbanks, G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J. Mol. Biol. 1976, 104, 243–261. [Google Scholar] [CrossRef]
- Grabarek, Z.; Gergely, J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Nakajima, N.; Ikada, Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous Media. Bioconj. Chem. 1995, 6, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Staros, J. V. Membrane-Impermeant Cross-Linking Reagents: Probes of the Structure and Dynamics of Membrane Proteins. Acc. Chem. Res. 1988, 21, 435–441. [Google Scholar] [CrossRef]
- Staros, J.V.; Wright, R.W.; Swingle, D.M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 1986, 156, 220–222. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; Prato, M. Preparation, functionalization and characterization of engineered carbon nanodots. Nat. Protoc. 2019, 14, 2931–2953. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Prado, A.R.; Keijok, W.J.; Ribeiro, M.R.N.; Pontes, M.J.; Nogueira, B.V.; Guimarães, M.C.C. A helpful method for controlled synthesis of monodisperse gold nanoparticles through response surface modeling. Arab. J. Chem. 2020. [Google Scholar] [CrossRef]
- Liz-marz, L.M. Nanometals: Formation and color. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Montenegro, J.; Grazu, V.; Sukhanova, A.; Agarwal, S.; De, J.M.; Nabiev, I.; Greiner, A.; Parak, W.J. Controlled antibody/(bio-) conjugation of inorganic nanoparticles for targeted delivery. Adv. Drug Deliv. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G. Bioconjugate Techniques, 2nd ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123705013. [Google Scholar]
- Bartczak, D.; Kanaras, A.G. Preparation of peptide-functionalized gold nanoparticles using one pot EDC/Sulfo-NHS coupling. Langmuir 2011, 27, 10119–10123. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, S.; Seher, A.; Trebing, J.; Weisenberger, D.; Rosenthal, A.; Siegmund, D.; Wajant, H. Fibroblast growth factor inducible (Fn14)-specific antibodies concomitantly display signaling pathway-specific agonistic and antagonistic activity. J. Biol. Chem. 2013, 288, 13455–13466. [Google Scholar] [CrossRef] [PubMed]

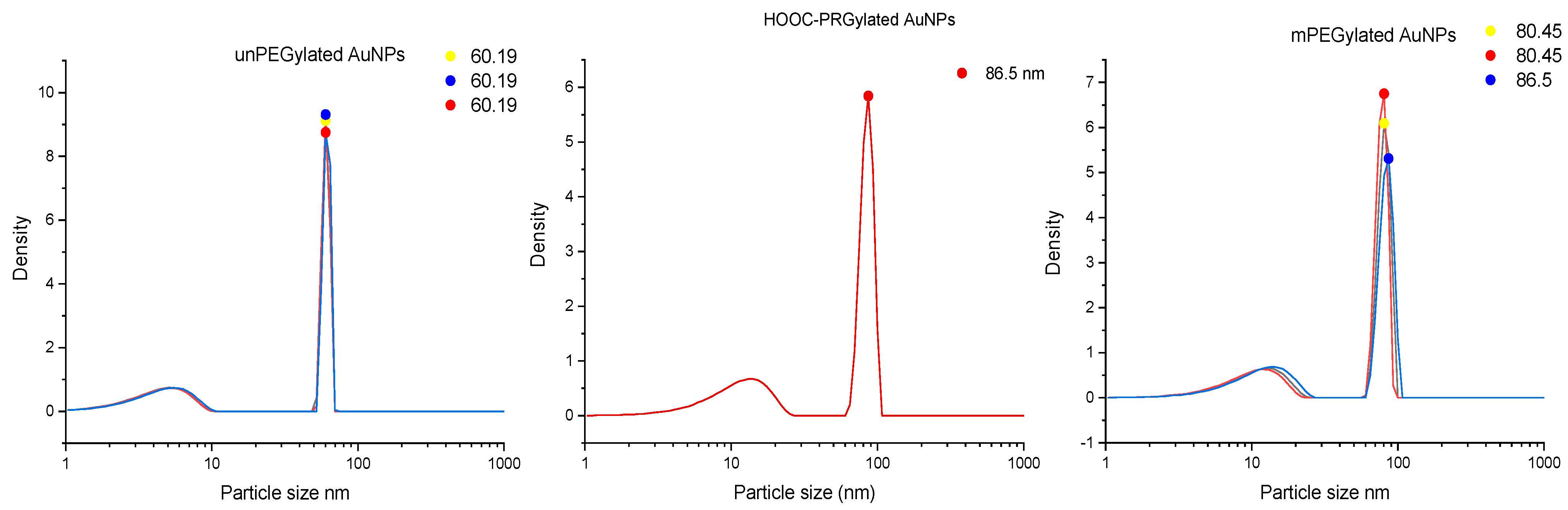

| Sample Structure | Particles Size | ζ Potential |
|---|---|---|
| Trisodium citrate—AuNPs | 60.19 nm | −14 mv |
| mPEG-AuNPs | 80.45 nm | −7 mv |
| HOOC-PEG-AuNPs | 86.5 nm | −20 mv |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aido, A.; Wajant, H.; Buzgo, M.; Simaite, A. Development of Anti-TNFR Antibody-Conjugated Nanoparticles. Proceedings 2021, 78, 55. https://doi.org/10.3390/IECP2020-08684
Aido A, Wajant H, Buzgo M, Simaite A. Development of Anti-TNFR Antibody-Conjugated Nanoparticles. Proceedings. 2021; 78(1):55. https://doi.org/10.3390/IECP2020-08684
Chicago/Turabian StyleAido, Ahmed, Harald Wajant, Matej Buzgo, and Aiva Simaite. 2021. "Development of Anti-TNFR Antibody-Conjugated Nanoparticles" Proceedings 78, no. 1: 55. https://doi.org/10.3390/IECP2020-08684
APA StyleAido, A., Wajant, H., Buzgo, M., & Simaite, A. (2021). Development of Anti-TNFR Antibody-Conjugated Nanoparticles. Proceedings, 78(1), 55. https://doi.org/10.3390/IECP2020-08684






