Partial Substitution of Fresh Microalgae with Baker’s Yeast (Saccharomyces cerevisiae) Enhances the Growth of Juvenile Ostrea edulis and Ruditapes decussatus †
Abstract
:1. Introduction
2. Materials and Methods
- (SGR) = 100 × ((lnW2 − lnW1)/t), where W1 and W2 are the initial and final weights (g) of the bivalves and t is the number of feeding days;
- (CI) = (flesh dry weight/shell dry weight) × 100.
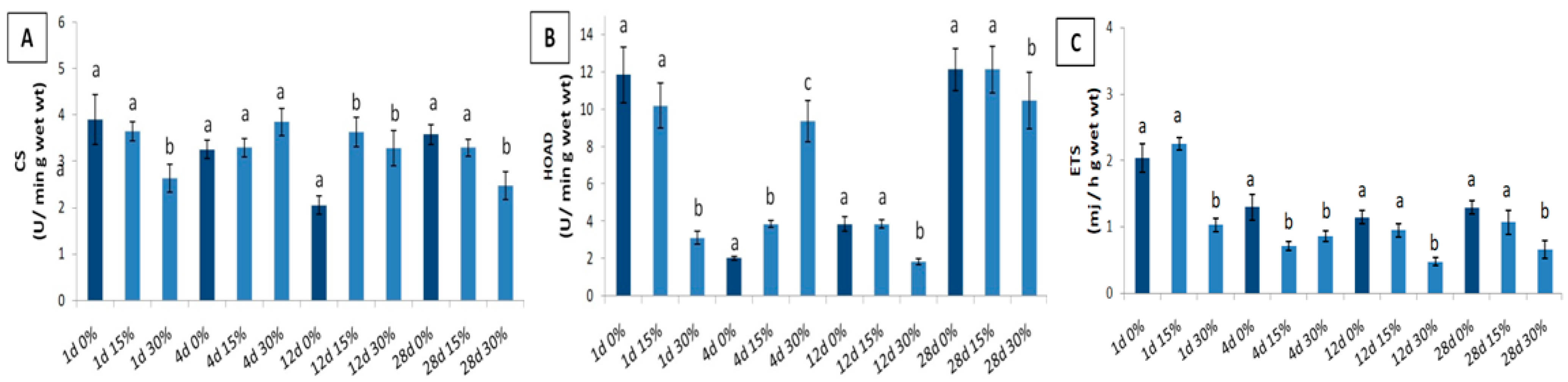
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coutteau, P.; Sorgeloos, P. Substitute diets for live algae in the intensive rearing of bivalve mollusks: A state of the art report. World Aquacult. 1993, 24, 45–52. [Google Scholar]
- Willer, D.F.; Aldridge, D.C. Microencapsulated diets to improve bivalve shellfish aquaculture for global food security. Glob. Food Secur. 2019, 23, 64–73. [Google Scholar] [CrossRef]
- Coutteau, P.; Hadley, N.H.; Manzi, J.J.; Sorgeloos, P. Effect of algal ration and substitution of algae by manipulated yeast diets on the growth of juvenile Mercenaria mercenaria. Aquaculture 1994, 120, 135–150. [Google Scholar] [CrossRef]
- Tanyaros, S.; Sujarit, C.; Jansri, N.; Tarangkoon, W. Baker’s yeast as a substitute for microalgae in the hatchery rearing of larval and juvenile tropical oyster (Crassostrea belcheri, Sowerby 1871). J. Appl. Aquacult. 2016, 28, 35–46. [Google Scholar] [CrossRef]
- Supono, S.; Mugica, M.; Spreitzenbarth, S.; Jeffs, A. Potential for Concentrated Microalgae as Replacement Diets for Juvenile Green-Lipped Mussels, Perna canaliculus. Aquacult. Res. 2023, 2023, 9841172. [Google Scholar] [CrossRef]
- Nell, J.A.; Sheridan, A.K.; Smith, I.R. Progress in a Sydney rock oyster, Saccostrea commercialis (Iredale and Roughley), breeding program. Aquaculture 1996, 144, 295–302. [Google Scholar] [CrossRef]
- Brown, M.R.; Barrett, S.M.; Volkman, J.K.; Nearhos, S.P.; Nell, J.A.; Allan, G.L. Biochemical composition of new yeasts and bacteria evaluated as food for bivalve aquaculture. Aquaculture 1996, 143, 341–360. [Google Scholar] [CrossRef]
- Irisarri, J.; Fernández-Reiriz, M.J.; Labarta, U. Temporal and spatial variations in proximate composition and Condition Index of mussels Mytilus galloprovincialis cultured in suspension in a shellfish farm. Aquaculture 2015, 435, 207–216. [Google Scholar] [CrossRef]
- Speers-Roesch, B.; Callaghan, N.I.; MacCormack, T.J.; Lamarre, S.G.; Sykes, A.V.; Driedzic, W.R. Enzymatic capacities of metabolic fuel use in cuttlefish (Sepia officinalis) and responses to food deprivation: Insight into the metabolic organization and starvation survival strategy of cephalopods. J. Comp. Physiol. B 2016, 186, 711–725. [Google Scholar] [CrossRef]
- Haider, F.; Sokolov, E.P.; Sokolova, I.M. Effects of mechanical disturbance and salinity stress on bioenergetics and burrowing behavior of the soft-shell clam Mya arenaria. J. Exp. Biol. 2018, 221, jeb172643. [Google Scholar] [CrossRef]
- Kolditz, C.; Borthaire, M.; Richard, N.; Corraze, G.; Panserat, S.; Vachot, C.; Lefèvre, F.; Médale, F. Liver and muscle metabolic changes induced by dietary energy content and genetic selection in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1154–R1164. [Google Scholar] [CrossRef] [PubMed]
- García-Esquivel, Z.; Bricelj, V.M.; González-Gómez, M.A. Physiological basis for energy demands and early postlarval mortality in the Pacific oyster, Crassostrea gigas. J. Exp. Mar. Biol. Ecol. 2001, 263, 77–103. [Google Scholar] [CrossRef]
- Loor, A.; Bossier, P.; Nevejan, N. Dietary substitution of microalgae with the Saccharomyces cerevisiae mutant, Δmnn9, for feeding Pacific oyster (Crassostrea gigas) juveniles. Aquaculture 2021, 534, 736253. [Google Scholar] [CrossRef]

| Ostrea edulis | Ruditapes decussatus | |||
|---|---|---|---|---|
| Condition index | mean | SD | mean | SD |
| Initial CI | 1.86 a | 0.24 | 18.35 a | 1.84 |
| Final CI—0% yeast | 2.05 a | 0.21 | 18.46 a | 1.78 |
| Final CI—15% yeast | 1.92 a | 0.12 | 18.31 a | 1.43 |
| Final CI—30% yeast | 1.94 a | 0.17 | 16.68 b | 0.98 |
| SGR of weight | mean | SD | mean | SD |
| SGRw 0% yeast | 0.102 a | 0.011 | 0.07 a | 0.005 |
| SGRw 15% yeast | 0.12 b | 0.008 | 0.108 b | 0.013 |
| SGRw 30% yeast | 0.085 c | 0.007 | 0.05 c | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, D.K.; Georgoulis, I.; Lattos, A.; Feidantsis, K.; Michaelidis, B.; Giantsis, I.A. Partial Substitution of Fresh Microalgae with Baker’s Yeast (Saccharomyces cerevisiae) Enhances the Growth of Juvenile Ostrea edulis and Ruditapes decussatus. Proceedings 2024, 94, 28. https://doi.org/10.3390/proceedings2024094028
Papadopoulos DK, Georgoulis I, Lattos A, Feidantsis K, Michaelidis B, Giantsis IA. Partial Substitution of Fresh Microalgae with Baker’s Yeast (Saccharomyces cerevisiae) Enhances the Growth of Juvenile Ostrea edulis and Ruditapes decussatus. Proceedings. 2024; 94(1):28. https://doi.org/10.3390/proceedings2024094028
Chicago/Turabian StylePapadopoulos, Dimitrios K., Ioannis Georgoulis, Athanasios Lattos, Konstantinos Feidantsis, Basile Michaelidis, and Ioannis A. Giantsis. 2024. "Partial Substitution of Fresh Microalgae with Baker’s Yeast (Saccharomyces cerevisiae) Enhances the Growth of Juvenile Ostrea edulis and Ruditapes decussatus" Proceedings 94, no. 1: 28. https://doi.org/10.3390/proceedings2024094028








