Abstract
A nanofibrous layer of polyvinylpyrrolidone (PVP) was designed to house, both in the fiber core and onto its outer surface, nanoparticles of mesoporous graphene (MGC), which are able to selectively adsorb acetic acid vapors. When grown on interdigital fingers microelectrodes (IDEs), upon UV-light irradiation taking place in air, the layer proved conductive and stable. Electrical and sensing features were significatively modulated by decorating the fiber surface with MGC (a sandwich-like structure) and polyethyleneimine (PEI). MGC, used both as a conductive filler and to decorate the fiber surface, strengthened the PVP scaffold and acted as a nucleation center for entrapping molecules of acetic acid. PEI improved the adhesion of MGC onto the surface. A preliminary study reported fast responses, high sensitivity with good linearity, selectivity, reversibility, and repeatability towards the acetic acid in ranges of up to hundreds of ppm at room temperature.
1. Introduction
Polyvinylpyrrolidone (PVP) is an eco-friendly and cost-effective polymer, which makes it a common choice for engineering nanoscale fine polymer fibers using electrospinning technology. However, due to its solubility in water and the most common organic solvents, it is too fragile to be considered a suitable matrix for gas-VOC chemical sensors. Nevertheless, UV-light irradiation can manipulate the PVP chain length, affecting the polymer modulus, tensile strength, and swelling, as well as providing it with new chemical-physical properties and a greater stability. In this project, electrospun and UV-treated PVP nanofibers (NFs) were functionalized with mesoporous graphitized carbon (MGC) nanopowder (with the dual function of the filler and surface binding site) and polyethyleneimine (PEI). This functionalization process aimed to develop a sensitive and selective sensor for acetic acid vapour [1], crucial in industrial settings where exposure to such vapours poses health risks to workers, potentially leadings to diseases contracted through prolonged contact with these materials (e.g., plastics, pharmaceuticals, dyes, insecticides and photographic chemicals industries). A sensor front-end circuit and a microcontroller unit (Arduino) were used for our experiments.
2. Materials and Methods
MGC (<500 nm), hexadecyltrimethylammonium bromide (CTAB), PVP (Mw: 1,300,000), PEI linear (Mw: 10,000), ethanol absolute (>98%), acetic acid (AcOH), formic acid, methanol (MeOH), dimethylformamide (DMF), acetone, cyclohexane (CyHex), triethylamine (TEA), and butylamine (ButA) were purchased from Merck and used without further purification. Interdigitated Electrodes (IDEs), provided by Micrux Technologies (Spain), were fabricated using borosilicate substrate (IDE sizes: 10 mm long, 6 mm wide, 0.75 mm thick, Pt/Ti electrodes, 120 pairs, 10 µm wide with 10 µm gap) and were subject to the electrospinning deposition of a PVP:MGC:CTAB homogeneous suspension in EtOH (1:0.003:0.01, wt), which occurred for two minutes (E: 4,6 kV, feed rate: 400 mL/h, d: 9 cm). Following UV-irradiation, sensors were dipped into an MGC-PEI-EtOH solution (0.0003:0.001:1, wt) and dried. Vapor measurements were carried out according to a dynamic mode at room temperature and a relative humidity percentage ranging between 40–50%.
3. Discussion
PVP fibers, following UV treatment, looked homogeneous and regular in both shape and size (d: ~350 nm). These fibers also present a rough surface (Figure 1A), which is likely due to the surfactant used for the stabilization of the MGC. After dipping, the fibrous network, as well as the substrate, appeared to be covered by a thin, very porous coating, which confirmed the PEI-MGC adhesion to the surface (Figure 1B). The mesoporous structure of MGC, used both as a conductive filler and to decorate the fiber surface, was expected to strengthen the PVP scaffold, which also acts as a nucleation center for entrapping molecules of acetic acid (AcOH) [1]. A thin film of polyethyleneimine (PEI) should provide a greater resistance of the fibers to humidity, as well as to increase adsorption sites for AcOH and improve the adhesion of MGC on the fibrous surface under the carrier gas flow. The number of dipping significantly modulates the electrical and sensing features. The selected sensor (R: 34 kΩ) was exposed to known concentrations of VOCs belonging to different chemical classes (Figure 1C). The shape curves of the transient responses indicated that the sensor responded quickly and selectively to acetic acid (Figure 1C). Thus, known amounts of vapors flowed through the measuring chamber and the resulting current changes are depicted in Figure 1C. The linear-like kinetics of the response to formic acid could be related to the participation of multiple sites of interactions. This includes possible lateral interactions between adsorbed molecules and multilayer formations, highlighting the different affinity of the sensor to the two organic acids. Thus, the sensor sensitivity to acetic acid was the highest response (Figure 1D) within the range investigated.
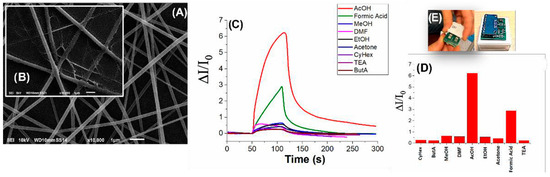
Figure 1.
SEM images of (A) PVP nanofibers housing MGC powder dispersion; (B) following decoration with PEI-MGC thin film; (C) transient sensor responses to common VOCs in dynamic modes, where DI is the current change and I0 the current baseline before VOC exposure; (D) bar plot depicting a comparison between the sensor responses to the same concentration of VOCs; (E) sensors and circuit sides of the sensing device.
Author Contributions
Conceptualization, A.M.; methodology, P.P., A.M. and E.Z.; validation, P.P.; investigation, P.P.; resources, A.M.; data curation, A.M., P.P., F.D.C., C.D.N. and G.T.; writing—original draft preparation, P.P. and A.M.; writing—review and editing, ALL the Authors; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Project “INAIL BRIC19 ID O7”—Integrated system of mobile and fixed sensors for dynamic spatio-temporal mapping of volatile compounds in work environments.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data that support the findings of this study are available after the reasonable request to the corresponding author.
Acknowledgments
Many thanks to A.R. Taddei for her SEM analysis, A. Capocecera for his technical collaboration and S. Berti and T. Davanzo for their administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
Reference
- Avossa, J.; Zampetti, E.; De Cesare, F.; Bearzotti, A.; Scarascia-Mugnozza, G.; Vitiello, G.; Zussman, E.; Macagnano, A. Thermally Driven Selective Nanocomposite PS-PHB/MGC Nanofibrous Conductive Sensor for Air Pollutant Detection. Front. Chem. 2018, 6, 432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).