Evaluating the Use of a Thermal Sensor to Detect Small Ground-Nesting Birds in Semi-Arid Environments during Winter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Licenses and Permits
2.3. Data Collection
2.4. Flight Procedures
2.4.1. Test Flights: 9 February 2023
2.4.2. Experimental Trials: 11 and 18 February 2023
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001. [Google Scholar] [CrossRef]
- Millsap, B.A.; Gore, J.A.; Runde, D.E.; Cerulean, S.I. Setting priorities for the conservation of fish and wildlife species in Florida. Wildl. Monogr. 1990, 3–57. [Google Scholar]
- Caveny, R.J.; Voelkel, S.J.; Brademan, W.T.; Hardin, J.B.; Peterson, M.J.; Collier, B.A. Distribution, fidelity, and abundance of Rio Grande wild turkey roosts in the Texas coastal sand plains. In Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies, Nashville, TN, USA, 22–26 October 2011; Volume 65, pp. 45–50. [Google Scholar]
- Guberti, V.; Stancampiano, L.; Ferrari, N. Surveillance, monitoring, and survey of wild life diseases: A public health and conservation approach. Hystrix. 2014, 25, 3–8. [Google Scholar]
- Schwarz, C.J.; Seber, G.A.F. Estimating animal abundance: Review III. Stat. Sci. 1999, 14, 427–456. [Google Scholar] [CrossRef]
- Iijima, H. A review of wildlife abundance estimation models: Comparison of models for correct application. Mammal. Study 2020, 45, 177–188. [Google Scholar] [CrossRef]
- Buckland, S.T.; Marsden, S.J.; Green, R.E. Estimating bird abundance: Making methods work. Bird Conserv. Int. 2008, 18, S91–S108. [Google Scholar] [CrossRef]
- Buckland, S.T. Point-transect surveys for songbirds: Robust methodologies. Auk 2006, 123, 345–357. [Google Scholar] [CrossRef]
- Howe, E.J.; Buckland, S.T.; Després-Einspenner, M.; Kühl, H.S. Distance sampling with camera traps. Methods Ecol. Evol. 2017, 8, 1558–1565. [Google Scholar] [CrossRef]
- Collier, B.A.; Ditchkoff, S.S.; Raglin, J.B.; Smith, J.M. Detection probability and sources of variation in white-tailed deer spotlight surveys. J. Wildl. Manag. 2007, 71, 277–281. [Google Scholar] [CrossRef]
- DeYoung, C.A. Accuracy of helicopter surveys of deer in south Texas. Wildl. Soc. Bull. 1985, 13, 146–149. [Google Scholar]
- Strobel, B.N.; Butler, M.J. Monitoring whooping crane abundance using aerial surveys: Influences on detectability. Wildl. Soc. Bull. 2013, 38, 188–195. [Google Scholar] [CrossRef]
- Chase, M.J.; Schlossberg, S.; Griffin, C.R.; Bouché, P.J.; Djene, S.W.; Elkan, P.W.; Ferreira, S.; Grossman, F.; Kohi, E.M.; Landen, K.; et al. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ 2016, 4, e2354. [Google Scholar] [CrossRef] [PubMed]
- Gentle, M.; Finch, N.; Speed, J.; Pople, A. A comparison of unmanned aerial vehicles (drones) and manned helicopters for monitoring macropod populations. Wildl. Res. 2018, 45, 586–594. [Google Scholar] [CrossRef]
- Kingsford, R.T.; Porter, J.L.; Brandis, K.J.; Ryall, S. Aerial surveys of waterbirds in Australia. Sci. Data 2020, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.R.; Gilliland, S.G.; Reed, E.T.; Roy, C. Comparing waterfowl densities detected through helicopter and airplane sea duck surveys in Labrador, Canada. Avian Conserv. Ecol. 2022, 17, 24. [Google Scholar] [CrossRef]
- Shupe, T.E.; Guthery, F.S.; Beasom, S.L. Use of helicopters to survey northern bobwhite populations on rangeland. Wildl. Soc. Bull. 1987, 15, 458–462. [Google Scholar]
- Rabe, M.J.; Rosenstock, S.S.; deVos, J.C., Jr. Review of big-game survey methods used by wildlife agencies of the western United States. Wildl. Soc. Bull. 2002, 30, 46–52. [Google Scholar]
- Stephenson, A. Parque Nacionale do Limpopo: Aerial Wildlife Census. Alan Stephenson Wildlife Management Services. 2010; p. 42. Available online: https://www.anac.gov.mz/wp-content/uploads/2017/07/PNL_Fixed-Wing-Census_Report-Oct-2010.pdf (accessed on 5 September 2023).
- DeYoung, C.A.; Guthery, F.S.; Beasom, S.L.; Coughlin, S.P.; Heffelfinger, J.R. Improving estimates of white-tailed deer abundance from helicopter surveys. Wildl. Soc. Bull. 1989, 17, 275–279. [Google Scholar]
- Bleich, V.C.; Bowyer, R.; Pauli, A.M.; Nicholson, M.C.; Anthes, R.W. Mountain sheep Ovis canadensis and helicopter surveys: Ramifications for the conservation of large mammals. Biol. Conserv. 1994, 70, 1–7. [Google Scholar] [CrossRef]
- Anderson, C.R., Jr. Lindzey, F.G. Moose sightability model developed from helicopter surveys. Wildl. Soc. Bull. 1996, 24, 247–259. [Google Scholar]
- Edwards, J.T.; Hernández, F.; Wester, D.B.; Brennan, L.A.; Parent, C.J. Effects of Native and Non-Native Invasive Grasses on Northern Bobwhite Habitat in South Texas. Rangel. Ecol. Manag. 2022, 84, 98–107. [Google Scholar] [CrossRef]
- Johnson, B.K.; Lindzey, F.G.; Guenzel, R.J. Use of aerial line transect surveys to estimate pronghorn populations in Wyoming. Wildl. Soc. Bull. 1991, 19, 315–321. [Google Scholar]
- Ward, C.L. Evaluation of Survey Techniques and Sightability for Pronghorn Antelope (Antilocapra americana) in Texas. Ph.D. Thesis, Texas A&M University-Kingsville, Kingsville, TX, USA, 2016. [Google Scholar]
- Kufeld, R.C.; Olterman, J.H.; Bowden, D.C. A helicopter quadrat census for mule deer on Uncompahgre Plateau, Colorado. J. Wildl. Manag. 1980, 44, 632. [Google Scholar] [CrossRef]
- Bodie, W.L.; Garton, E.O.; Taylor, E.R.; McCoy, M. A sightability model for bighorn sheep in canyon habitats. J. Wildl. Manag. 1995, 59, 832. [Google Scholar] [CrossRef]
- Rusk, J.P.; Hernández, F.; Arredondo, J.A.; Hernández, F.; Bryant, F.C.; Hewitt, D.G.; Redeker, E.J.; Brennan, L.A.; Bingham, R.L. An Evaluation of Survey Methods for Estimating Northern Bobwhite Abundance in Southern Texas. J. Wildl. Manag. 2007, 71, 1336–1343. [Google Scholar] [CrossRef]
- DeMaso, S.J.; Guthery, F.S.; Spears, G.S.; Rice, S.M. Morning covey calls as an index of northern bobwhite density. Wildl. Soc. Bull. 1992, 20, 94–101. [Google Scholar]
- Guthery, F.S.; Crews, A.K.; Lusk, J.J.; Chapman, R.N.; Sams, M. Effects of bag limits on bobwhite hunters and harvest. J. Wildl. Manag. 2004, 68, 1095–1103. [Google Scholar] [CrossRef]
- Woodard, D.A.; A Brennan, L.; Hernández, F.; Perotto-Baldivieso, H.L.; Wilkins, N.; Montalvo, A. Evaluating the harvest rate recommendation for northern bobwhites in South Texas. Natl. Quail Symp. Proc. 2022, 9, 56. [Google Scholar] [CrossRef]
- Sasse, D.B. Job-related mortality of wildlife workers in the United States, 1937–2000. Wildl. Soc. Bull. 2003, 31, 1015–1020. [Google Scholar]
- Durkin, P. The Leading Cause of Wildlife Biologists Deaths. Conservation/Wildlife Management, The Meateater. 2021. Available online: https://www.themeateater.com/conservation/wildlife-management/the-leading-cause-of-wildlife-biologist-deaths (accessed on 5 September 2023).
- Fust, P.; Loos, J. Development perspectives for the application of autonomous, unmanned aerial systems (UASs) in wildlife conservation. Biol. Conserv. 2019, 241, 108380. [Google Scholar] [CrossRef]
- Camacho, A.M.; Perotto-Baldivieso, H.L.; Tanner, E.P.; Montemayor, A.L.; Gless, W.A.; Exum, J.; Yamashita, T.J.; Foley, A.M.; DeYoung, R.W.; Nelson, S.D. The broad scale impact of climate change on planning aerial wildlife surveys with drone-based thermal cameras. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, L.; Théau, J.; Ménard, P. Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Beaver, J.T.; Baldwin, R.W.; Messinger, M.; Newbolt, C.H.; Ditchkoff, S.S.; Silman, M.R. Evaluating the use of drones equipped with thermal sensors as an effective method for estimating wildlife. Wildl. Soc. Bull. 2020, 44, 434–443. [Google Scholar] [CrossRef]
- Blum, J.; Foley, A.M.; DeYoung, R.W.; Hewitt, D.G.; Baungardt, J.; Hellickson, M.W.; Perotto-Baldivieso, H.L. Evaluation of drone surveys for ungulates in southwestern rangelands. Wildl. Soc. Bull. 2024, in press. [Google Scholar]
- Ivanova, S.; Prosekov, A.; Kaledin, A. A survey on monitoring of wild animals during fires using drones. Fire 2022, 5, 60. [Google Scholar] [CrossRef]
- Burke, C.; Rashman, M.F.; Longmore, S.N.; McAree, O.; Glover-Kapfer, P.; Ancrenaz, M.; Wich, S.A. Successful observation of orangutans in the wild with thermal-equipped drones. J. Unmanned Veh. Syst. 2019, 7, 235–257. [Google Scholar] [CrossRef]
- Burke, C.; Rashman, M.; Wich, S.; Symons, A.; Theron, C.; Longmore, S. Optimizing observing strategies for monitoring animals using drone-mounted thermal infrared cameras. Int. J. Remote. Sens. 2019, 40, 439–467. [Google Scholar] [CrossRef]
- Gazagne, E.; Gray, R.J.; Ratajszczak, R.; Brotcorne, F.; Hambuckers, A. Unmanned aerial vehicles (UAVs) with thermal infrared (TIR) sensors are effective for monitoring and counting threatened Vietnamese primates. Primates 2023, 64, 407–413. [Google Scholar] [CrossRef]
- Bird, C.N.; Dawn, A.H.; Dale, J.; Johnston, D.W. A Semi-Automated Method for Estimating Adélie Penguin Colony Abundance from a Fusion of Multispectral and Thermal Imagery Collected with Unoccupied Aircraft Systems. Remote Sens. 2020, 12, 3692. [Google Scholar] [CrossRef]
- Chen, A.; Jacob, M.; Shoshani, G.; Charter, M. Using computer vision, image analysis and UAVs for the automatic recognition and counting of common cranes (Grus grus). J. Environ. Manag. 2023, 328, 116948. [Google Scholar] [CrossRef]
- Shewring, M.P.; Vafidis, J.O. Using UAV-mounted thermal cameras to detect the presence of nesting nightjar in upland clear-fell: A case study in South Wales, UK. Ecol. Solut. Évid. 2021, 2, e12052. [Google Scholar] [CrossRef]
- Santangeli, A.; Chen, Y.; Kluen, E.; Chirumamilla, R.; Tiainen, J.; Loehr, J. Integrating drone-borne thermal imaging with artificial intelligence to locate bird nests on agricultural land. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Karp, D. Detecting small and cryptic animals by combining thermography and a wildlife detection dog. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- E Martin, M.; A Hearon, L.; O Evans, K.; Iglay, R.B.; I Morrison, J.; McConnell, M.D. Detection Rates of Northern Bobwhite Coveys Using a Small Unmanned Aerial System-Mounted Thermal Camera. Natl. Quail Symp. Proc. 2022, 9, 51. [Google Scholar] [CrossRef]
- Perry, M.C.; Lehmann, V.W. Bobwhites in the Rio Grande Plain of Texas. J. Wildl. Manag. 1986, 50, 521. [Google Scholar] [CrossRef]
- Burger, L.W.; Miller, D.A.; Southwick, R.I. Economic impact of northern bobwhite hunting in the southeastern United States. Wildl. Soc. Bull. 1999, 27, 1010–1018. [Google Scholar]
- Hernandez, F.; Guthery, F.S. Beef, Brush, and Bobwhites: Quail Management in Cattle Country, 2nd ed.; Texas A&M University Press: College Station, TX, USA, 2017. [Google Scholar]
- Brennan, L.A.; Kuvlesky, W.P., Jr. North American grassland birds: An unfolding conservation crisis? J. Wildl. Manag. 2005, 69, 1–13. [Google Scholar] [CrossRef]
- Brennan, L.A.; Williford, D.L.; Ballard, B.M.; Kuvlesky, W.P.; Grahmann, E.D.; DeMaso, S.J. The Upland and Webless Migratory Game Birds of Texas; Texas A&M University Press: College Station, TX, USA, 2017. [Google Scholar]
- Brennan, L.A.; Tanner, A.; Tanner, E.P. Adaptive management and quail conservation on rangelands in the American West. Natl. Quail Symp. Proc. 2022, 9, 8. [Google Scholar] [CrossRef]
- Crosby, A.D.; Elmore, R.D.; Leslie, D.M., Jr.; Will, R.E. Looking beyond rare species as umbrella species: Northern Bobwhites (Colinus virginianus) and conservation of grassland and shrubland birds. Biol. Conserv. 2015, 186, 233–240. [Google Scholar] [CrossRef]
- Hernández, F.; Brennan, L.A.; DeMaso, S.J.; Sands, J.P.; Wester, D.B. On reversing the northern bobwhite population decline: 20 years later. Wildl. Soc. Bull. 2012, 37, 177–188. [Google Scholar] [CrossRef]
- U.S. Climate Data. 2023. Available online: https://www.usclimatedata.com/climate/kingsville/texas/united-states/ustx0697 (accessed on 2 July 2023).
- Rosene, W. The Bobwhite Quail, Its Life and Management; Rutgers University Press: New Brunswick, NJ, USA, 1969. [Google Scholar]
- Case, R.M. Bioenergetics of a covey of bobwhites. Wilson Bull. 1973, 85, 52–59. [Google Scholar]
- Williams, C.K.; Lutz, R.; Applegate, R.D. Optimal group size and northern bobwhite coveys. Anim. Behav. 2003, 66, 377–387. [Google Scholar] [CrossRef]
- Read, D.R.; Senzig, D.A.; Cutler, C.J.; Elmore, E.; He, H. Noise Measurement Report: Unconventional Aircraft-Choctaw Nation of Oklahoma: July 2019 (No. DOT-VNTSC-FAA-20-03); John, A., Ed.; Volpe National Transportation Systems Center (US): Cambridge, MA, USA, 2020. [Google Scholar]
- Teledyne FLIR. Picking a Thermal Color Palette. Teledyne Flir. 2023. Available online: https://www.flir.com/discover/industrial/picking-a-thermal-color-palette/ (accessed on 20 September 2023).
- Teledyne FLIR. DELTA Episode 5—Isotherms. Teledyne FLIR. 2023. Available online: https://www.flir.com/suas/delta/delta-episode-5/ (accessed on 30 September 2023).
- McKinley, R.L.; Mills, C.N. A comparison of several goodness-of-fit statistics. Appl. Psychol. Meas. 1985, 9, 49–57. [Google Scholar] [CrossRef]
- Preacher, K.J. Calculation for the Chi-Square Test: An Interactive Calculation Tool for chi-Square Tests of Goodness of Fit and Independence [Computer software]. 2001. Available online: http://quantpsy.org (accessed on 12 September 2023).
- McKnight, P.E.; Najab, J. Kruskal-wallis test. Corsini Encycl. Psychol. 2010, 1, 1–10. [Google Scholar]
- Ostertagova, E.; Ostertag, O.; Kováč, J. Methodology and application of the Kruskal-Wallis test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- St, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar]
- McKnight, P.E.; Najab, J. Mann-Whitney U Test. Corsini Encycl. Psychol. 2010. [Google Scholar] [CrossRef]
- Rebolo-Ifrán, N.; Graña Grilli, M.; Lambertucci, S.A. Drones as a Threat to Wildlife: YouTube Complements Science in Providing Evidence about Their Effect. Environ. Conserv. 2019, 46, 205–210. [Google Scholar] [CrossRef]
- Frye, O.E., Jr. The comparative survival of wild and pen-reared bobwhite in the field. In Proceedings of the Transactions of the North American Wildlife Conference, Toronto, ON, Canada, 8–10 April 1942; Volume 7, pp. 168–178. [Google Scholar]
- Roseberry, J.L.; Ellsworth, D.L.; Klimstra, W.D. Comparative post-release behavior and survival of wild, semi-wild, and game farm bobwhites. Wildl. Soc. Bull. 1987, 15, 449–455. [Google Scholar]
- Reyna, K.S.; Newman, W.L. Comparative analysis of behavioural response of captive-reared and wild-trapped northern bobwhites to simulated predator attacks. Avian Biol. Res. 2018, 11, 16–23. [Google Scholar] [CrossRef]
- Perkins, R.; Boal, C.; Rollins, D.; Perez, R.M. Northern bobwhite predator avoidance behavior in response to varying types of threat. J. Wildl. Manag. 2014, 78, 1272–1281. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote. Sens. 2018, 40, 407–419. [Google Scholar] [CrossRef]
- Havens, K.J.; Sharp, E.J. Thermal Imaging Techniques to Survey and Monitor Animals in the Wild: A Methodology; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Klimstra, W.D.; Ziccardi, V.C. Night-roosting habitat of bobwhites. J. Wildl. Manag. 1963, 27, 202. [Google Scholar] [CrossRef]
- Chavarria, P.M.; Kocek, A.R.; Silvy, N.J.; Lopez, R.R. Use of portable infrared cameras to facilitate detection and capture success of Montezuma quail. Natl. Quail Symp. Proc. 2017, 7, 122. [Google Scholar] [CrossRef]
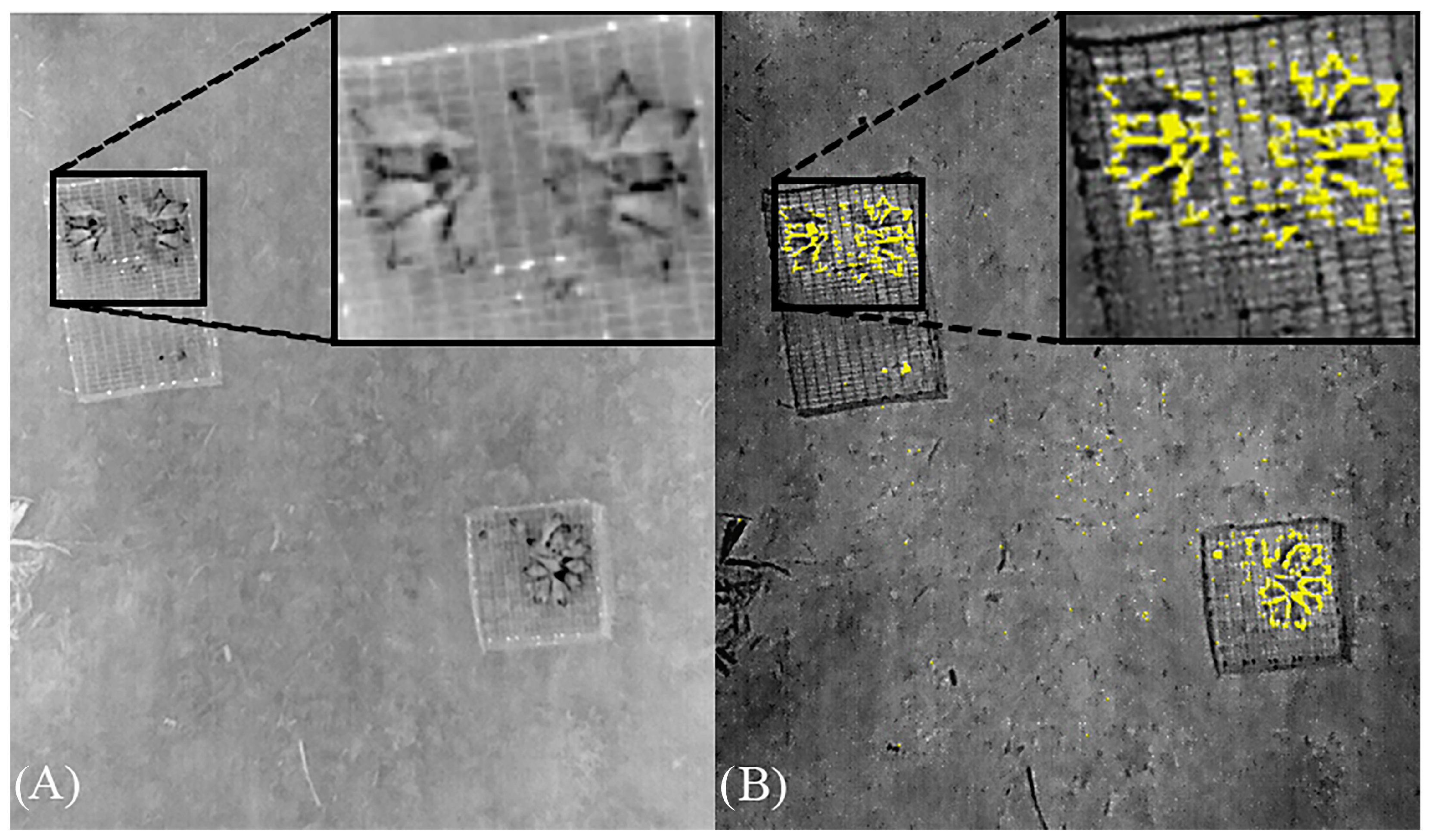

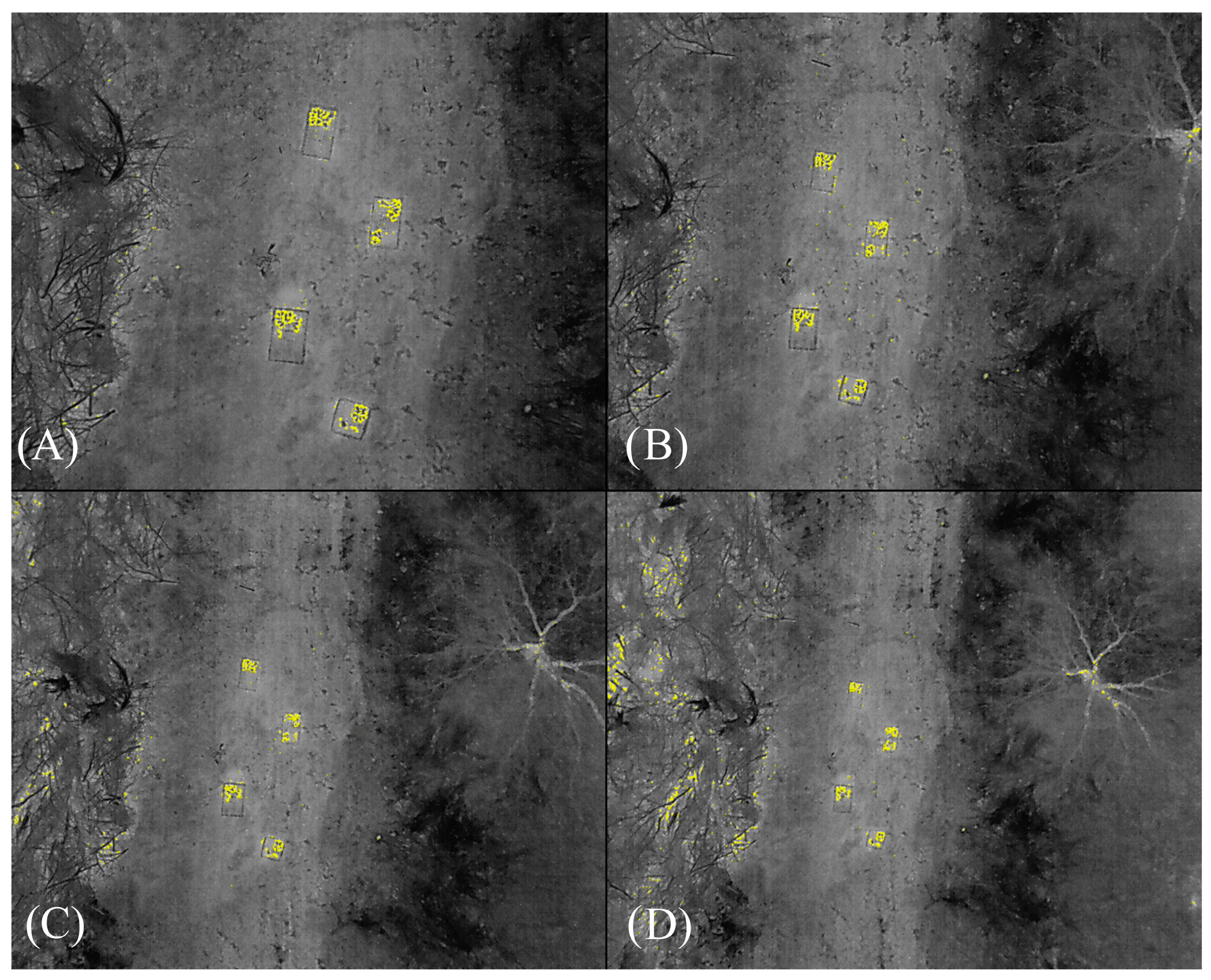
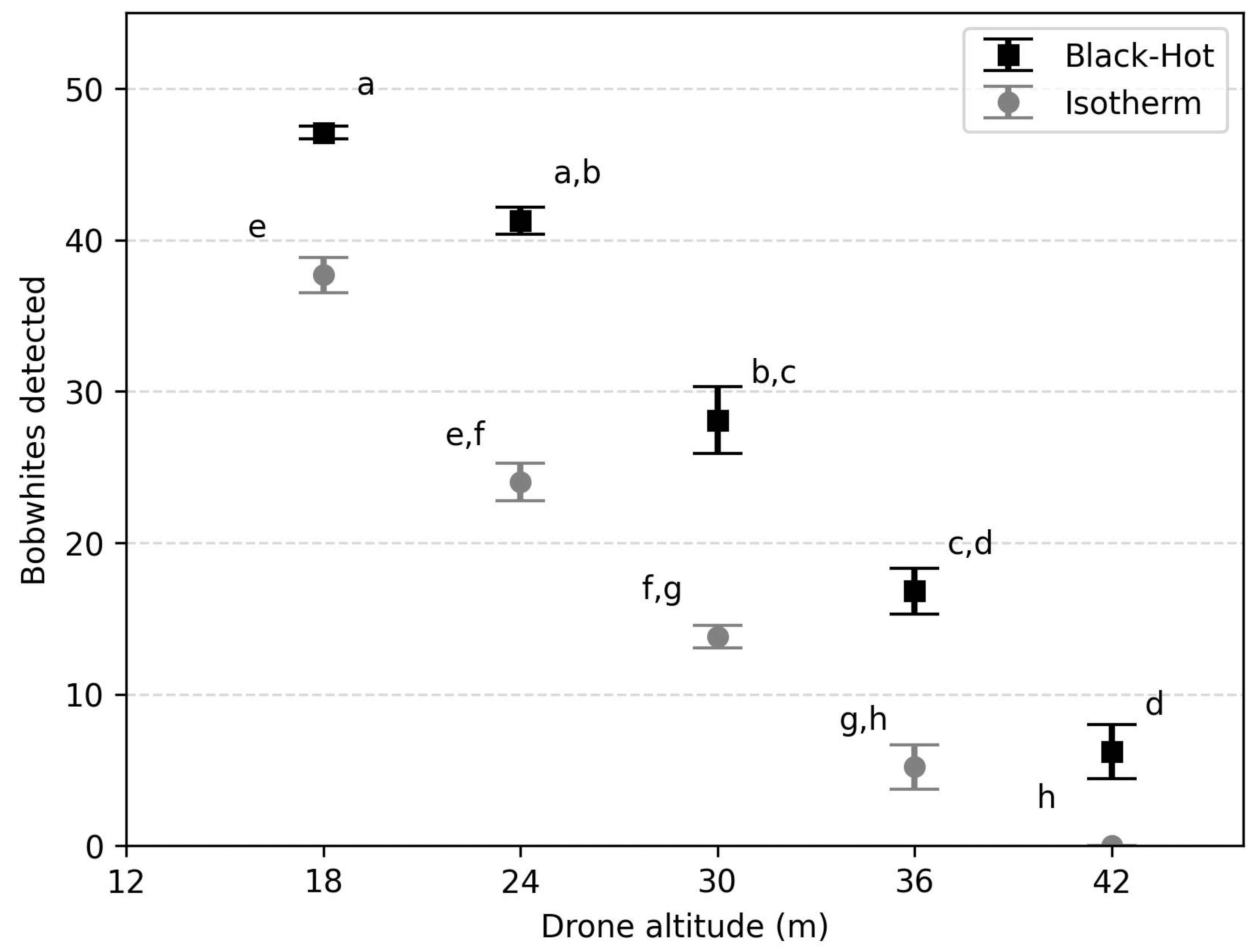
| Date (Trial) | Temperature Kingsville, TX. NAS Weather Station | Study Site (°C) | Wind Speed (m/s) | Sunset (h) | Start Time (h) | Isotherm Interval Threshold (°C) | |||
|---|---|---|---|---|---|---|---|---|---|
| High (°C) | Low (°C) | Upper | Middle | Lower | |||||
| (Test) | 24.4 | 3.9 | 5.5 | <2.2 | 18:19 | 20:00 | 26.2 | 20.5 | 15.1 |
| (1) | 19.4 | 1.1 | 8.9 | <2.6 | 18:21 | 19:45 | 26.2 | 20.5 | 15.1 |
| (2) | 20.0 | 2.8 | 11.3 | <1.0 | 18:26 | 19:50 | 31.7 | 25.5 | 20.0 |
| Drone Altitude (m) | Pixel Size (cm) | Image Footprint (m2) | # Images Required to Cover One Hectare |
|---|---|---|---|
| 61 | 5.32 | 1012.08 | 10 |
| 55 | 4.80 | 822.21 | 13 |
| 49 | 4.28 | 653.90 | 16 |
| 42 | 3.66 | 479.60 | 21 |
| 36 | 3.14 | 352.16 | 29 |
| 30 | 2.62 | 244.98 | 41 |
| 24 | 2.09 | 156.70 | 64 |
| 18 | 1.57 | 88.35 | 114 |
| Cage | Number of Bobwhites | ||
|---|---|---|---|
| Test Flights | Experimental Flights 1 | Experimental Flights 2 | |
| A | 12 | 14 | 14 |
| B | 11 | 13 | 13 |
| C | 8 | 12 | 12 |
| D | 8 | 12 | 12 |
| E | 6 | - | - |
| F | 6 | - | - |
| Drone Altitude (m) | Black-Hot | Isotherm | ||
|---|---|---|---|---|
| Chi-Square (df = 9) | p-Value | Chi-Square (df = 9) | p-Value | |
| 18 | 3.27 | 0.952 | 68.10 | <0.001 |
| 24 | 19.86 | 0.019 | 145.65 | <0.001 |
| 30 | 111.47 | <0.001 | 272.35 | <0.001 |
| 36 | 233.29 | <0.001 | 415.14 | <0.001 |
| 42 | 399.06 | <0.001 | 510.00 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Sanchez, J.S.; Perotto-Baldivieso, H.L.; Massey, L.D.; Ortega-S., J.A.; Brennan, L.A.; Hernández, F. Evaluating the Use of a Thermal Sensor to Detect Small Ground-Nesting Birds in Semi-Arid Environments during Winter. Drones 2024, 8, 64. https://doi.org/10.3390/drones8020064
Avila-Sanchez JS, Perotto-Baldivieso HL, Massey LD, Ortega-S. JA, Brennan LA, Hernández F. Evaluating the Use of a Thermal Sensor to Detect Small Ground-Nesting Birds in Semi-Arid Environments during Winter. Drones. 2024; 8(2):64. https://doi.org/10.3390/drones8020064
Chicago/Turabian StyleAvila-Sanchez, J. Silverio, Humberto L. Perotto-Baldivieso, Lori D. Massey, J. Alfonso Ortega-S., Leonard A. Brennan, and Fidel Hernández. 2024. "Evaluating the Use of a Thermal Sensor to Detect Small Ground-Nesting Birds in Semi-Arid Environments during Winter" Drones 8, no. 2: 64. https://doi.org/10.3390/drones8020064





