Estimating Total Length of Partially Submerged Crocodylians from Drone Imagery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Equipment
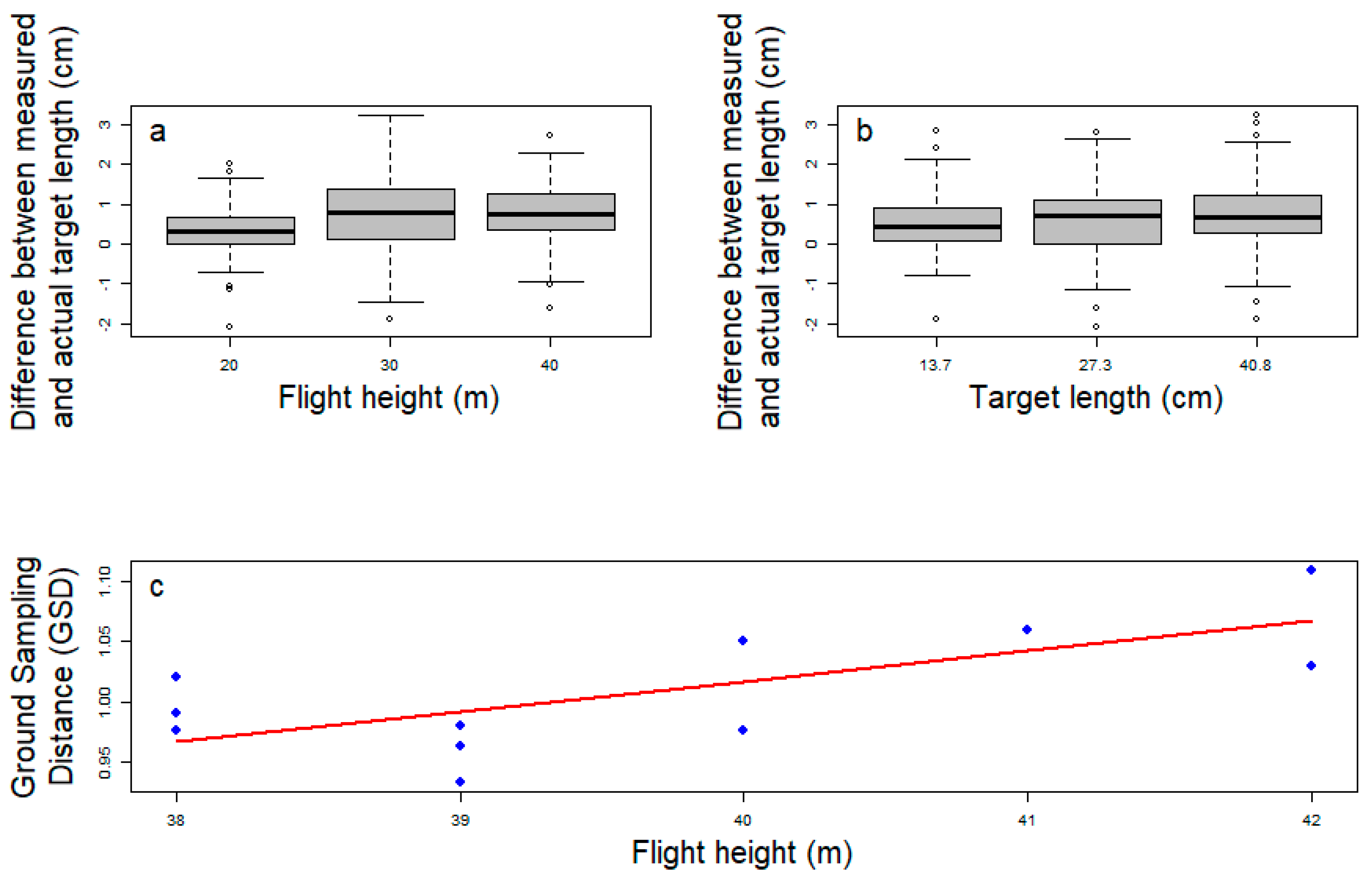
2.2. Calibration of Flight and Photo Parameters for Optimal Measurements
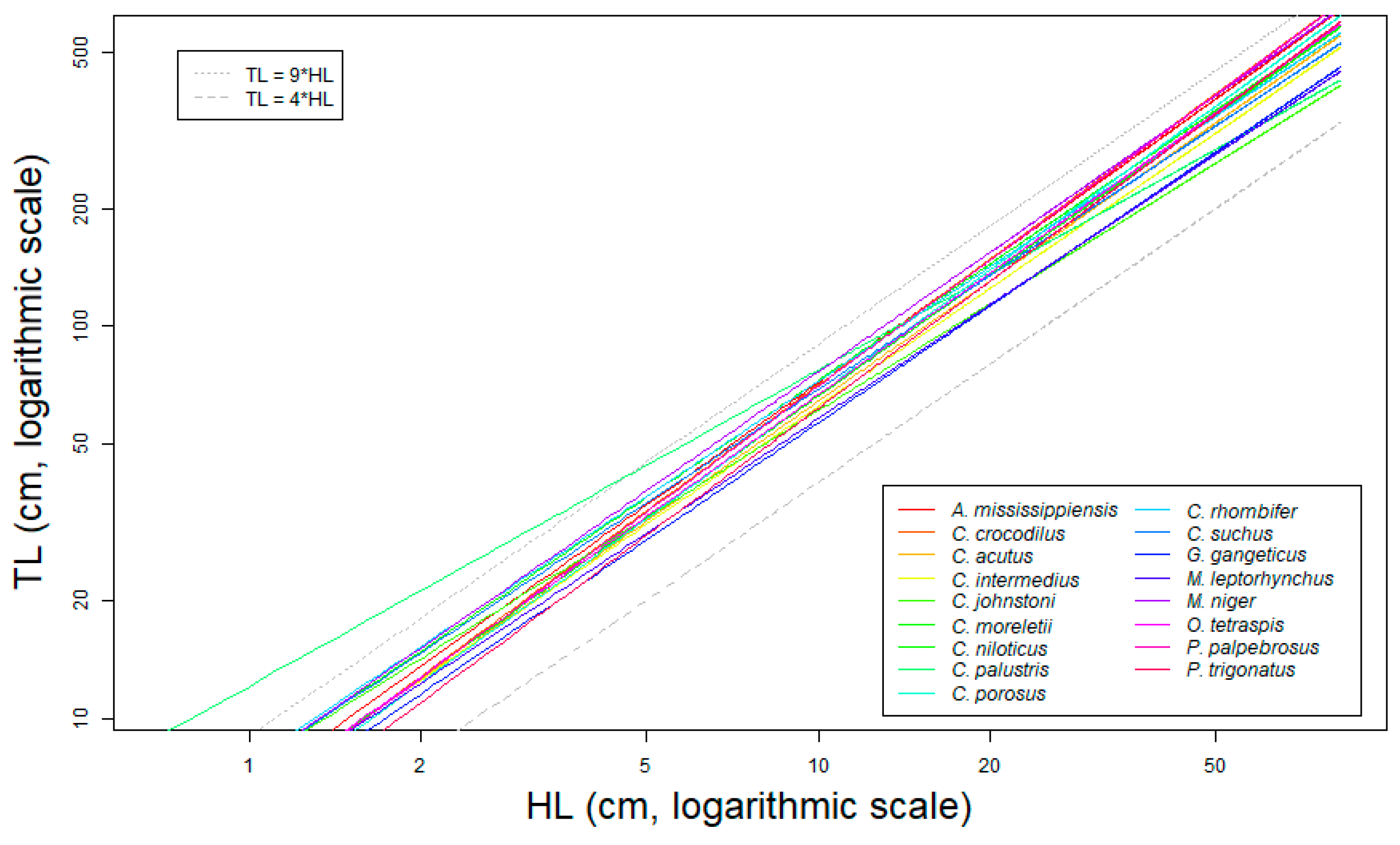
2.3. Allometric Ratios for Total Length Determination
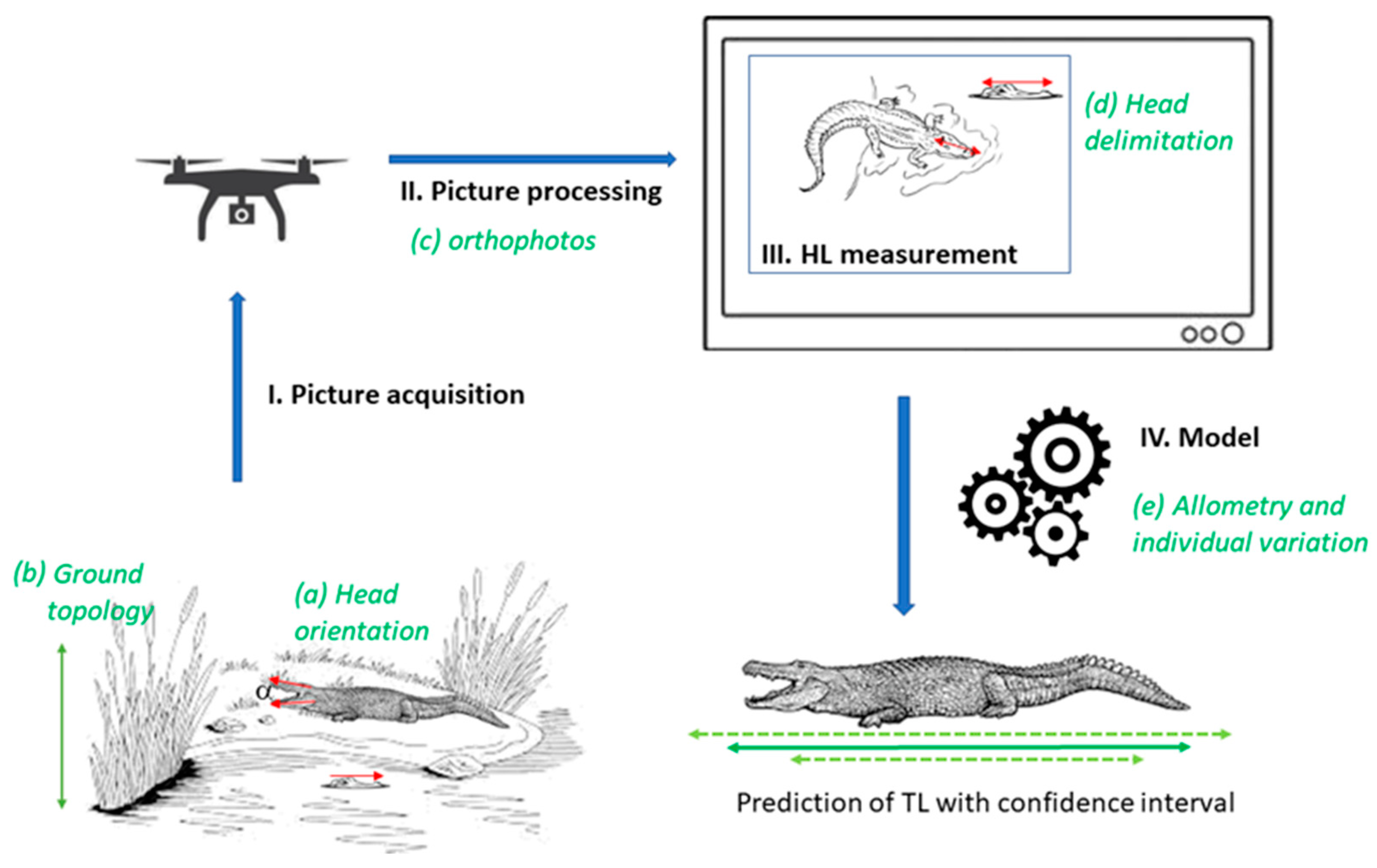
- Head inclination: because the drone camera objective is vertically oriented, direct estimation of the HL from the picture implicitly assumes that the head is horizontally oriented. In reality, head inclination can deviate from the horizontal plane due to the terrain slope, because crocodylians thermoregulate by opening their mouths, or when they are simply resting at any non-horizontal angle. This leads to an underestimation of the real HL by a cos(θ) factor, where θ is the angle between the head inclination and the horizontal plane (Figure 1). We simulated head inclination using a distribution for [0°; 90°] (Figure S1a). Since we have no data to fit that inclination, we arbitrarily chose the distribution parameters so that the average inclination equals 5° and that θ < 20° for 99% of the samples, a conservative choice.
- Target length estimation: we compared the lengths measured in drone photos lengths (HLe) to the know lengths (HL0) of the mock targets. The imprecision of the HLe measurement () can result from variation of the distance between the ground and the drone altitude (due to topology), orthophoto treatment, or observer accuracy in choosing the two reference points (i.e., head delimitation effect; Figure 1). We measured this imprecision as and fitted a Johnson’s SU-distribution, a 4-parameter distribution that is more flexible than the classical normal distribution. In particular, this distribution can be asymmetric. We used the logarithm of the relative error, rather than the absolute error, to stabilize the variance (heteroscedasticity). We tried both Gaussian and Johnson’s SU distributions, where goodness-of-fit indicated that Johnson’s SU better fit the data (Figure S1b).
- Allometry: to take into account the natural variability of individuals and the limited size of the sample of allometry data above, we used a simple linear regression ln(TL) = f[ln(HL)] to predict TL from HL, and to estimate the confidence interval around TL for a given HL. The logarithm is used to stabilize the residual variance, in accordance with the standard hypothesis of the linear model. Overall, the total imprecision on the total body length prediction (TLe) is thus the consequence of all these independent sources of imprecision (head inclination, target length acquisition, and allometry). We simulated them 50 times each to produce the overall confidence intervals around TLe, thereby establishing a robust reference allometric framework. We then determined the part of the total deviance of TLe from TL explained by each source using an ANOVA. We performed all analyses in R version 4.2.2 [51].
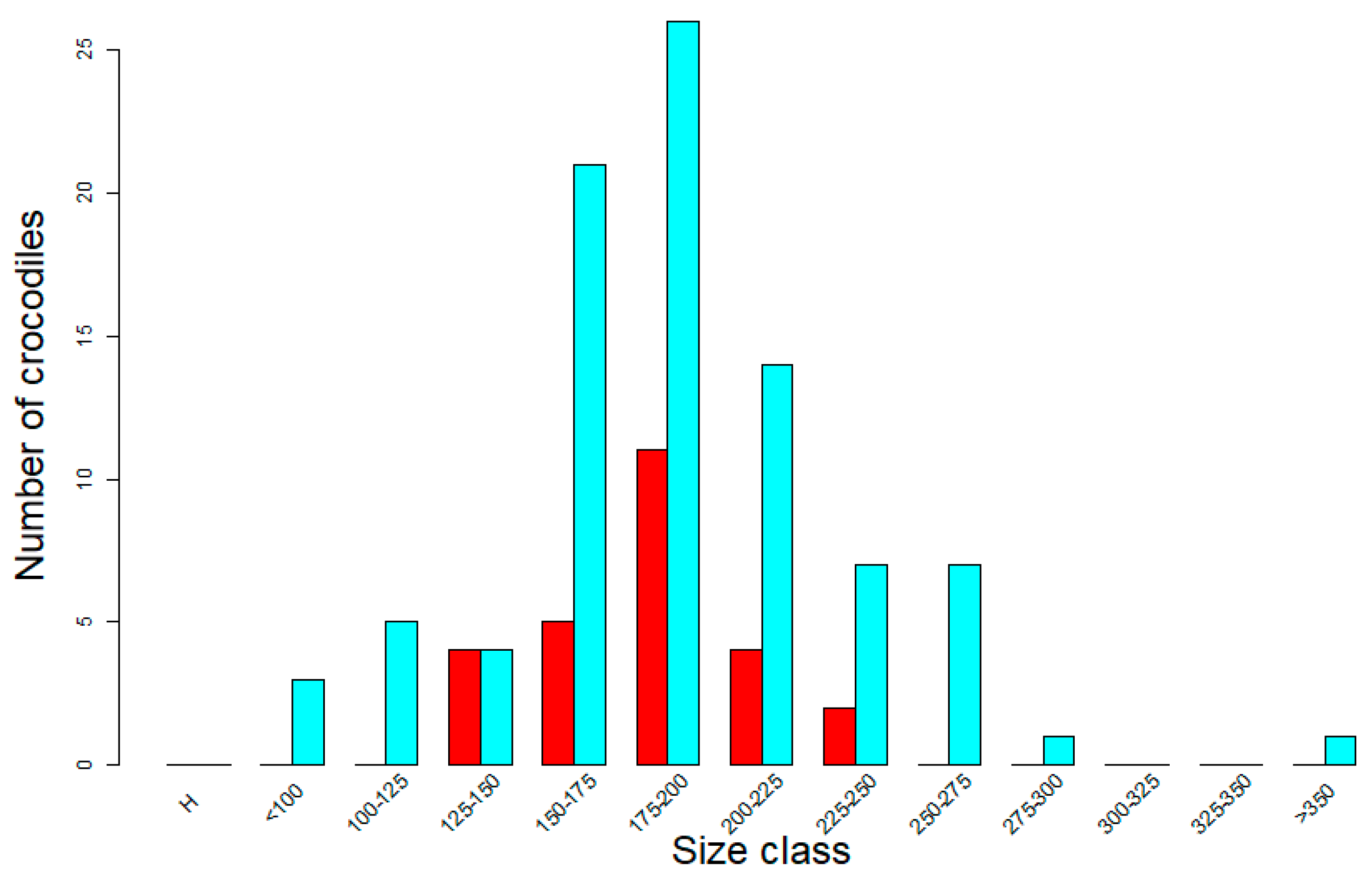
2.4. Crocodile Size Class Distribution in Natural Populations
3. Results and Discussion
3.1. Drone-Captured Pictures Allow Precise Target Length Measurement
3.2. Reference Allometric Framework for Estimating Total Length from Head Length in Crocodylians
- Measurement bias: We accounted for the measurement imprecision in drone photos previously identified from the standard targets by using a Johnson’s SU-distribution, which better fit the data than a Gaussian distribution (Figure S1b). The Johnson’s SU-distribution was fitted on the logarithm of the relative measurement error and the value of its four parameters are: gamma = 0.0947, delta = 0.936, xi = 0.0209 and lambda = 0.0227.
- Head inclination: The drone objective is perpendicular to the ground, thus if the target is not horizontal its size can be underestimated (see Methods, Figure 1). This could be particularly problematic to measure crocodile head length because crocodylians often incline their head. We assessed this potential distortion by conservatively assuming that, on average, crocodiles have a head inclination of 5° and 99% of the population have a head inclination < 20° (Figure S1a; pers. obs.). With this assumption, we calculated that we underestimate the true length in drone photos by 0.7% on average, and that the underestimate is less than 6% for 99% of the population. Randomly adding target inclination distortions in our model further confirmed that it results in limited relative imprecision (2.7% of total variability).
- Allometric variation: For all species, we observed a robust allometric relationship between HL and TL (See Table 1, Figure 5a and Figures S2a–S17a). Our data show that the absolute variation of the allometric relationship increases with the size of the individuals (i.e., more variance around the predicted values for bigger crocodylians), but with a fairly constant relative error (average ≃ 9.63%, range ≃5.8% for M. leptorhynchus to ≃15% for G. gangeticus, not including C. palustris, which we excluded because of the afore-explained data quality problems). As it was directly measured on real crocodiles, this variation comes from biological processes independent from the measuring method.
3.3. Improved Demographic Classification of Wild Crocodile Populations from Drones—But with Limitations
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Segaran, R.R.; Reid, I.; Terauds, A.; Koh, L.P. Drones Count Wildlife More Accurately and Precisely than Humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Wirsing, A.J.; Johnston, A.N.; Kiszka, J.J.; Wirsing, A.J.; Johnston, A.N.; Kiszka, J.J. Foreword to the Special Issue on ‘The Rapidly Expanding Role of Drones as a Tool for Wildlife Research’. Wildl. Res. 2022, 49, i–v. [Google Scholar] [CrossRef]
- McEvoy, J.F.; Hall, G.P.; McDonald, P.G. Evaluation of Unmanned Aerial Vehicle Shape, Flight Path and Camera Type for Waterfowl Surveys: Disturbance Effects and Species Recognition. PeerJ 2016, 4, e1831. [Google Scholar] [CrossRef]
- Floreano, D.; Wood, R.J. Science, Technology and the Future of Small Autonomous Drones. Nature 2015, 521, 460–466. [Google Scholar] [CrossRef]
- Ventura, D.; Bruno, M.; Jona Lasinio, G.; Belluscio, A.; Ardizzone, G. A Low-Cost Drone Based Application for Identifying and Mapping of Coastal Fish Nursery Grounds. Estuar. Coast. Shelf Sci. 2016, 171, 85–98. [Google Scholar] [CrossRef]
- Ogden, L.E. Drone Ecology. BioScience 2013, 63, 776. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Dandois, J.P.; Holl, K.D.; Nadwodny, D.; Reid, J.L.; Ellis, E.C. Using Lightweight Unmanned Aerial Vehicles to Monitor Tropical Forest Recovery. Biol. Conserv. 2015, 186, 287–295. [Google Scholar] [CrossRef]
- Koh, L.P.; Wich, S.A. Dawn of Drone Ecology: Low-Cost Autonomous Aerial Vehicles for Conservation. Trop. Conserv. Sci. 2012, 5, 121–132. [Google Scholar] [CrossRef]
- Vas, E.; Lescroël, A.; Duriez, O.; Boguszewski, G.; Grémillet, D. Approaching Birds with Drones: First Experiments and Ethical Guidelines. Biol. Lett. 2015, 11, 20140754. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Moguédec, G.L.; Assio, C.; Blatrix, R.; Ahizi, M.N.; Hedegbetan, G.C.; Kpera, N.G.; Lapeyre, V.; Martin, D.; Labbé, P.; et al. Evaluation of the Use of Drones to Monitor a Diverse Crocodylian Assemblage in West Africa. Wildl. Res. 2021, 49, 11–23. [Google Scholar] [CrossRef]
- Schofield, G.; Katselidis, K.A.; Lilley, M.K.S.; Reina, R.D.; Hays, G.C. Detecting Elusive Aspects of Wildlife Ecology Using Drones: New Insights on the Mating Dynamics and Operational Sex Ratios of Sea Turtles. Funct. Ecol. 2017, 31, 2310–2319. [Google Scholar] [CrossRef]
- Adame, K.; Pardo, M.A.; Salvadeo, C.; Beier, E.; Elorriaga-Verplancken, F.R. Detectability and Categorization of California Sea Lions Using an Unmanned Aerial Vehicle. Mar. Mammal Sci. 2017, 33, 913–925. [Google Scholar] [CrossRef]
- Shah, K.; Ballard, G.; Schmidt, A.; Schwager, M. Multidrone Aerial Surveys of Penguin Colonies in Antarctica. Sci. Robot. 2020, 5, eabc3000. [Google Scholar] [CrossRef] [PubMed]
- Rahman, D.A.; Herliansyah, R.; Subhan, B.; Hutasoit, D.; Imron, M.A.; Kurniawan, D.B.; Sriyanto, T.; Wijayanto, R.D.; Fikriansyah, M.H.; Siregar, A.F.; et al. The First Use of a Photogrammetry Drone to Estimate Population Abundance and Predict Age Structure of Threatened Sumatran Elephants. Sci. Rep. 2023, 13, 21311. [Google Scholar] [CrossRef] [PubMed]
- Penny, S.; White, R.; Scott, D.; MacTavish, L.; Pernetta, A. Using Drones and Sirens to Elicit Avoidance Behaviour in White Rhinoceros as an Anti-Poaching Tactic. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191135. [Google Scholar] [CrossRef] [PubMed]
- Reischig, T.; Resende, E.; Cordes, H. Controlling Poaching of Nesting Loggerhead Sea Turtles with Night Vision Unmanned Aerial Vehicles on Boavista Island, Cabo Verde. Afr. Sea Turtle Newsl. 2018, 10, 9–13. [Google Scholar]
- Shirley, M.H.; Eaton, M.J. Procédures Standard de Suivi des Populations de Crocodiles; Groupe Spécialiste de Crocodiles: Darwin, Australia, 2012. [Google Scholar]
- Webb, G.; Manolis, S.C.; Whitehead, P.J. Wildlife Management: Crocodiles and Alligators; S. Beatty & Sons/Conservation Commission of the Northern Territory: Chipping Norton, Australia, 1987; ISBN 978-0-949324-09-2. [Google Scholar]
- Thorbjarnarson, J.; Platt, S.G.; Khaing, U.S.T. A Population Survey of the Estuarine Crocodile in the Ayeyarwady Delta, Myanmar. Oryx 2000, 34, 317–324. [Google Scholar] [CrossRef]
- Shirley, M.H.; Oduro, W.; Beibro, H.Y. Conservation Status of Crocodiles in Ghana and Côte-d’Ivoire, West Africa. Oryx 2009, 43, 136–145. [Google Scholar] [CrossRef]
- Fukuda, Y.; Saalfeld, K.; Webb, G.; Manolis, C.; Risk, R. Standardised Method of Spotlight Surveys for Crocodiles in the Tidal Rivers of the Northern Territory, Australia. North. Territ. Nat. 2013, 24, 14–32. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Pienaar, D. Degradation of the Crocodile Population in the Olifants River Gorge of Kruger National Park, South Africa. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 155–164. [Google Scholar] [CrossRef]
- Shirley, M.H.; Dorazio, R.M.; Abassery, E.; Elhady, A.A.; Mekki, M.S.; Asran, H.H. A Sampling Design and Model for Estimating Abundance of Nile Crocodiles While Accounting for Heterogeneity of Detectability of Multiple Observers. J. Wildl. Manag. 2012, 76, 966–975. [Google Scholar] [CrossRef]
- Martin, J.; Edwards, H.H.; Burgess, M.A.; Percival, H.F.; Fagan, D.E.; Gardner, B.E.; Ortega-Ortiz, J.G.; Ifju, P.G.; Evers, B.S.; Rambo, T.J. Estimating Distribution of Hidden Objects with Drones: From Tennis Balls to Manatees. PLoS ONE 2012, 7, e38882. [Google Scholar] [CrossRef]
- Elsey, R.M.; Trosclair, P.L. The Use of an Unmanned Aerial Vehicle to Locate Alligator Nests. Southeast. Nat. 2016, 15, 76–82. [Google Scholar] [CrossRef]
- Marín-Enríquez, E.; Charruau, P.; Félix-Salazar, L.A. Discovery of a Suburban Wetland Refuge for a Depleted American Crocodile (Crocodylus Acutus) Population in Northwestern Mexico, Using a Commercial Unmanned Aerial Vehicle. Trop. Conserv. Sci. 2023, 16, 19400829231209848. [Google Scholar] [CrossRef]
- Scarpa, L.J.; Piña, C.I. The Use of Drones for Conservation: A Methodological Tool to Survey Caimans Nests Density. Biol. Conserv. 2019, 238, 108235. [Google Scholar] [CrossRef]
- Harvey, K.R.; Hill, G.J.E. Mapping the Nesting Habitats of Saltwater Crocodiles (Crocodylus porosus) in Melacca Swamp and the Adelaide River Wetlands, Northern Territory: An Approach Using Remote Sensing and GIS. Wildl. Res. 2003, 30, 365–375. [Google Scholar] [CrossRef]
- Evans, I.J.; Jones, T.H.; Pang, K.; Evans, M.N.; Saimin, S.; Goossens, B. Use of Drone Technology as a Tool for Behavioral Research: A Case Study of Crocodilian Nesting. Herpetol. Conserv. Biol. 2015, 10, 90–98. [Google Scholar]
- Evans, L.J.; Jones, T.H.; Pang, K.; Saimin, S.; Goossens, B. Spatial Ecology of Estuarine Crocodile (Crocodylus porosus) Nesting in a Fragmented Landscape. Sensors 2016, 16, 1527. [Google Scholar] [CrossRef] [PubMed]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring Behavioral Responses of Sea Turtles, Saltwater Crocodiles, and Crested Terns to Drone Disturbance to Define Ethical Operating Thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef]
- Desai, B.; Patel, A.; Patel, V.; Shah, S.; Raval, M.S.; Ghosal, R. Identification of Free-Ranging Mugger Crocodiles by Applying Deep Learning Methods on UAV Imagery. Ecol. Inform. 2022, 72, 101874. [Google Scholar] [CrossRef]
- Sawan, S.; Mondal, T.; Williams, A.C.; Yadav, S.P.; Krishnamurthy, R. Hybrid Drone-Based Survey of Riverine Habitat and Crocodiles in Complex Landscapes. Int. J. Environ. Sci. Technol. 2023, 20, 13571–13582. [Google Scholar] [CrossRef]
- Ezat, M.A.; Fritsch, C.J.; Downs, C.T. Use of an Unmanned Aerial Vehicle (Drone) to Survey Nile Crocodile Populations: A Case Study at Lake Nyamithi, Ndumo Game Reserve, South Africa. Biol. Conserv. 2018, 223, 76–81. [Google Scholar] [CrossRef]
- Jordaan, P.R. The Establishment of a Multifaceted Crocodylus Niloticus Laurenti 1768 Monitoring Programme on Maputo Special Reserve (Maputo Province, Mozambique) with Preliminary Notes on the Population (Reptilia: Crocodylidae). Herpetol. Notes 2021, 14, 1155–1162. [Google Scholar]
- Woolcock, A.B.; Cotton, S.; Cotton, A.J. Effectiveness of Using Drones and Convolutional Neural Networks to Monitor Aquatic Megafauna. Afr. J. Ecol. 2022, 60, 544–556. [Google Scholar] [CrossRef]
- Thapa, G.J.; Thapa, K.; Thapa, R.; Jnawali, S.R.; Wich, S.A.; Poudyal, L.P.; Karki, S. Counting Crocodiles from the Sky: Monitoring the Critically Endangered Gharial (Gavialis gangeticus) Population with an Unmanned Aerial Vehicle (UAV). J. Unmanned Veh. Syst. 2018, 6, 71–82. [Google Scholar] [CrossRef]
- Nichols, J.D. Population Models and Crocodile Management; Surrey Beatty and Sons: Chipping Norton, NSW, Australia, 1987. [Google Scholar]
- Webb, G.J.; Smith, A.M. Life History Parameters, Population Dynamics and the Management of Crocodilians. In Wildlife Management: Crocodiles and Alligators; Surrey Beatty: Sydney, Australia, 1987; pp. 199–210. [Google Scholar]
- Da Silveira, R.; Magnusson, W.E.; Campos, Z. Monitoring the Distribution, Abundance and Breeding Areas of Caiman Crocodilus Crocodilus and Melanosuchus Niger in the Anavilhanas Archipelago, Central Amazonia, Brazil. J. Herpetol. 1997, 31, 514–520. [Google Scholar] [CrossRef]
- Combrink, X.; Warner, J.; Hofmeyr, M.; Govender, D.; Ferreira, S. Standard Operating Procedure for the Monitoring, Capture and Sampling of Nile Crocodiles (Crocodylus niloticus); Unpublished report; South African National Parks: Skukuza, South Africa, 2013. [Google Scholar]
- Coulson, A.R.; Hernandez, T. Alligator Metabolism Studies on Chemical Reactions In Vivo. Comp. Biochem. Physiol. Part B Comp. Biochem. 1983, 74, 1–175. [Google Scholar] [CrossRef]
- Bennett, A.F.; Seymour, R.; Bradford, D.F.; Webb, G. Mass-Dependence of Anaerobic Metabolism and Acid-Base Disturbance during Activity in the Salt-Water Crocodile, Crocodylus porosus. J. Exp. Biol. 1985, 118, 161–171. [Google Scholar] [CrossRef]
- Seymourl, R.S.; Webb, G.J.; Albert, F. Effect of Capture on the Physiology of Crocodylus porosus; Surrey Beatty and Sons: Chipping Norton, NSW, Australia, 1987. [Google Scholar]
- Franklin, C.E.; Davis, B.M.; Peucker, S.K.J.; Stephenson, H.; Mayer, R.; Whittier, J.; Lever, J.; Grigg, G.C. Comparison of Stress Induced by Manual Restraint and Immobilisation in the Estuarine Crocodile, Crocodylus porosus. J. Exp. Zoolog. A Comp. Exp. Biol. 2003, 298A, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, H.G.; Anderson, G.S.; Gruny, L.; Sperou, E.S.; Heard, D.J. Use of Blood Lactate in Assessment of Manual Capture Techniques of Zoo-Housed Crocodilians. Animals 2022, 12, 397. [Google Scholar] [CrossRef]
- Choquenot, D.; Webb, G.J.W. A Photographic Technique for Estimating the Size of Crocodiles Seen in Spotlight Surveys and for Quantifying Observer Bias. In Wildlife Management: Crocodiles and Alligators; Surrey Beatty and Sons: Chipping Norton, NSW, Australia, 1987; pp. 217–224. [Google Scholar]
- Magnusson, W.E. Size Estimates of Crocodilians. J. Herpetol. 1983, 17, 86–88. [Google Scholar] [CrossRef]
- Fukuda, Y.; Saalfeld, K.; Lindner, G.; Nichols, T. Estimation of Total Length from Head Length of Saltwater Crocodiles (Crocodylus porosus) in the Northern Territory, Australia. J. Herpetol. 2013, 47, 34–40. [Google Scholar] [CrossRef]
- Wawrzyn, D. What Is Ground Sample Distance and How Does It Affect Your Drone Data? Available online: https://www.propelleraero.com/blog/ground-sample-distance-gsd-calculate-drone-data/ (accessed on 27 July 2023).
- R Core Team R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. 2023.
- Brisson-Curadeau, É.; Bird, D.; Burke, C.; Fifield, D.A.; Pace, P.; Sherley, R.B.; Elliott, K.H. Seabird Species Vary in Behavioural Response to Drone Census. Sci. Rep. 2017, 7, 17884. [Google Scholar] [CrossRef] [PubMed]
- Rush, G.P.; Clarke, L.E.; Stone, M.; Wood, M.J. Can Drones Count Gulls? Minimal Disturbance and Semiautomated Image Processing with an Unmanned Aerial Vehicle for Colony-Nesting Seabirds. Ecol. Evol. 2018, 8, 12322–12334. [Google Scholar] [CrossRef] [PubMed]
- Bennitt, E.; Bartlam-Brooks, H.L.A.; Hubel, T.Y.; Wilson, A.M. Terrestrial Mammalian Wildlife Responses to Unmanned Aerial Systems Approaches. Sci. Rep. 2019, 9, 2142. [Google Scholar] [CrossRef] [PubMed]
- Linchant, J.; Lhoest, S.; Quevauvillers, S.; Lejeune, P.; Vermeulen, C.; Semeki Ngabinzeke, J.; Luse Belanganayi, B.; Delvingt, W.; Bouché, P. UAS Imagery Reveals New Survey Opportunities for Counting Hippos. PLoS ONE 2018, 13, e0206413. [Google Scholar] [CrossRef] [PubMed]
- Palomino-González, A.; Kovacs, K.M.; Lydersen, C.; Ims, R.A.; Lowther, A.D. Drones and Marine Mammals in Svalbard, Norway. Mar. Mammal Sci. 2021, 37, 1212–1229. [Google Scholar] [CrossRef]
- Mo, M.; Bonatakis, K. Approaching Wildlife with Drones: Using Scientific Literature to Identify Factors to Consider for Minimising Disturbance. Aust. Zool. 2021, 42, 1–29. [Google Scholar] [CrossRef]
- Ross, J.P.; Crocodile Specialist Group. In Proceedings of the International Workshop for Management and Trade of Caiman Yacare, Gainsville, FL, USA, 3–5 October 2002. 2003. Available online: http://aquaticcommons.org/id/eprint/2568 (accessed on 5 August 2023).
- Platt, S.G.; Rainwater, T.R.; Finger, A.G.; Thorbjarnarson, J.B.; Anderson, T.A.; McMurry, S.T. Food Habits, Ontogenetic Dietary Partitioning and Observations of Foraging Behaviour of Morelet’s Crocodile (Crocodylus moreletii) in Northern Belize. Herpetol. J. 2006, 16, 281–290. [Google Scholar]
- Padilla, S.E.; González-Jáuregui, M.; Von Osten, J.R.; Valdespino, C.; López Luna, M.A.; Quiróz, G.B.; Barão-Nóbrega, J.A.L. Using Regression Tree Analysis to Determine Size Class Intervals and Sexual Dimorphism in the Morelet’s Crocodile Crocodylus moreletii. Wildl. Biol. 2020, 2020, wlb.00707. [Google Scholar] [CrossRef]
- Montague, J. Morphometric Analysis of Crocodylus Novaeguineae from the Fly River Drainage, Papua New Guinea. Wildl. Res. 1984, 11, 395. [Google Scholar] [CrossRef]
- Kushlan, J.A.; Mazzotti, F.J. Population Biology of the American Crocodile. J. Herpetol. 1989, 23, 7–21. [Google Scholar] [CrossRef]
- Platt, S.G.; Thorbjarnarson, J.B. Status and Conservation of the American Crocodile, Crocodylus acutus, in Belize. Biol. Conserv. 2000, 96, 13–20. [Google Scholar] [CrossRef]
- Fukuda, Y.; Manolis, C.; Appel, K. Featured Article: Management of Human-Crocodile Conflict in the Northern Territory, Australia: Review of Crocodile Attacks and Removal of Problem Crocodiles. J. Wildl. Manag. 2014, 78, 1239–1249. [Google Scholar] [CrossRef]
- Grigg, G.C.; Seebacherd, F.; Beard, L.A.; Morris, D. Thermal Relations of Large Crocodiles, Crocodylus porosus, Free—Ranging in a Naturalistic Situation. Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1793–1799. [Google Scholar] [CrossRef]
- Downs, C.T.; Greaver, C.; Taylor, R. Body Temperature and Basking Behaviour of Nile Crocodiles (Crocodylus niloticus) during Winter. J. Therm. Biol. 2008, 33, 185–192. [Google Scholar] [CrossRef]
- Webb, G.; Manolis, C. Crocodiles of Australia; Reed Books Pty, Ltd.: Frenchs Forest, Australia, 1989. [Google Scholar]
- Montague, J.J. A New Size Record for the Saltwater Crocodile (Crocodylus porosus). Herpetol. Rev. 1983, 14, 36–37. [Google Scholar]
- Woodward, A.R.; White, J.H.; Linda, S.B. Maximum Size of the Alligator (Alligator mississippiensis). J. Herpetol. 1995, 29, 507–513. [Google Scholar] [CrossRef]
- Platt, S.G.; Rainwater, T.R.; Thorbjarnarson, J.B.; Finger, A.G.; Anderson, T.A.; McMurry, S.T. Size Estimation, Morphometrics, Sex Ratio, Sexual Size Dimorphism, and Biomass of Morelet’s Crocodile in Northern Belize. Caribb. J. Sci. 2009, 45, 80–93. [Google Scholar] [CrossRef]
- Britton, A.; Whitaker, R.; Whitaker, N. Here Be a Dragon: Exceptional Size in a Saltwater Crocodile (Crocodylus porosus) from the Philippines. Herpetol. Rev. 2012, 43, 541–546. [Google Scholar]
- Hutton, J.M. Morphometrics and Field Estimation of the Size of the Nile Crocodile. Afr. J. Ecol. 1987, 25, 225–230. [Google Scholar] [CrossRef]
- Whitaker, R.; Whitaker, N. Who’s Got the Biggest? Crocodile Specialist Group Newsletter: Darwin, Australia, 2008. [Google Scholar]
- Eaton, M.J.; Martin, A.; Thorbjarnarson, J.; Amato, G. Species-Level Diversification of African Dwarf Crocodiles (Genus Osteolaemus): A Geographic and Phylogenetic Perspective. Mol. Phylogenet. Evol. 2009, 50, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.K.; Combrink, X.; Calverley, P.; Champion, G.; Downs, C.T. Morphometrics, Sex Ratio, Sexual Size Dimorphism, Biomass, and Population Size of the Nile Crocodile (Crocodylus niloticus) at Its Southern Range Limit in KwaZulu-Natal, South Africa and James Hennessy from the The National Reptile Zoo, Ireland. Zoomorphology 2016, 135, 511–521. [Google Scholar] [CrossRef]
- Mobaraki, A.; Abtin, E.; Erfani, M.; Stevenson, C. Total Length and Head Length Relationship in Mugger Crocodiles Crocodylus Palustris (Reptilia: Crocodilia: Crocodylidae) in Iran. J. Threat. Taxa 2021, 13, 19162–19164. [Google Scholar] [CrossRef]
- Verdade, L.M. Regression Equations between Body and Head Measurements in the Broad-Snouted Caiman (Caiman latirostris). Rev. Bras. Biol. 2000, 60, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Webb, G.J.W.; Messel, H. Morphometric Analysis of Crocodylus porosus from the North Coast of Arnhem Land, Northern Australia. Aust. J. Zool. 1978, 26, 1–27. [Google Scholar] [CrossRef]
- Shirley, M.H.; Eaton, M.J. Africa Regional Reports: Trip Report: Niger 2007. Crocodile Spec. Group Newsl. 2008, 273, 21–23. [Google Scholar]
- Shirley, M.H.; Eaton, M.J. Niger Trip Report 2007. Unpublished Project Report, University of Florida.
- Thorbjarnarson, J.B. Ecology of the American Crocodile, Crocodylus acutus. In Proceedings of the 7th Working Meeting of the Crocodile Specialist Group of the Species Survival Commission of the International Union for Conservation of Nature and Natural Resources, Caracas, Venezuela, 21–28 October 1986; Volume 21, pp. 228–259. [Google Scholar]
- Somaweera, R.; Brien, M.; Shine, R. The Role of Predation in Shaping Crocodilian Natural History. Herpetol. Monogr. 2013, 27, 23–51. [Google Scholar] [CrossRef]
- Ouedraogo, I.; Oueda, A.; Hema, M.E.; Shirley, M.H.; Kabre, B.G. Impact of Anthropogenic Activities on the Abundance of Crocodylus Suchus (Saint-Hilaire 1807) within the Nazinga Game Ranch, Burkina Faso. Open J. Ecol. 2022, 12, 788–803. [Google Scholar] [CrossRef]
- Hutchings, J.A. Adaptive Life Histories Effected by Age-Specific Survival and Growth Rate. Ecology 1993, 74, 673–684. [Google Scholar] [CrossRef]
- Gaillard, J.-M.; Festa-Bianchet, M.; Yoccoz, N.G. Population Dynamics of Large Herbivores: Variable Recruitment with Constant Adult Survival. Trends Ecol. Evol. 1998, 13, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Caswell, H. Prospective and Retrospective Perturbation Analyses: Their Roles in Conservation Biology. Ecology 2000, 81, 619–627. [Google Scholar] [CrossRef]
- Saether, B.-E.; Coulson, T.; Festa-Bianchet, M.; Gaillard, J.-M.; Jenkins, A.; Jones, C.; Nicoll, M.A.C.; Norris, K.; Oli, M.K.; Ozgul, A.; et al. How Life History Influences Population Dynamics in Fluctuating Environments. Life Hist. Influ. Popul. Dyn. Fluctuating Environ. 2013, 182, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.R.; Moore, C.T. Use of crocodilian night count data for population trend estimation. In Proceedings of the 2nd Conference Crocodile Specialist Group Species Survival Commission, Darwin, NT, USA, 12–19 March 1993; pp. 12–13. [Google Scholar]
- Combrink, A.S. Population Survey of Crocodylus niloticus (Nile Crocodile) at Lake Sibaya, Republic of South Africa; Centre for Environment and Development, School of Applied Environmental Sciences, University of KwaZulu Natal: Durban, South Africa, 2004. [Google Scholar]
- Adoni, W.Y.H.; Lorenz, S.; Fareedh, J.S.; Gloaguen, R.; Bussmann, M. Investigation of Autonomous Multi-UAV Systems for Target Detection in Distributed Environment: Current Developments and Open Challenges. Drones 2023, 7, 263. [Google Scholar] [CrossRef]
- Seebacher, F.; Grigg, G.C. Patterns of Body Temperature in Wild Freshwater Crocodiles, Crocodylus Johnstoni: Thermoregulation versus Thermoconformity, Seasonal Acclimatization, and the Effect of Social Interactions. Copeia 1997, 1997, 549–557. [Google Scholar] [CrossRef]
- Myburgh, A.; Botha, H.; Downs, C.T.; Woodborne, S.M. The Application and Limitations of a Low-Cost UAV Platform and Open-Source Software Combination for Ecological Mapping and Monitoring. Afr. J. Wildl. Res. 2021, 51, 166–177. [Google Scholar] [CrossRef]
- Hua, A.; Martin, K.; Shen, Y.; Chen, N.; Mou, C.; Sterk, M.; Reinhard, B.; Reinhard, F.F.; Lee, S.; Alibhai, S.; et al. Protecting Endangered Megafauna through AI Analysis of Drone Images in a Low-Connectivity Setting: A Case Study from Namibia. PeerJ 2022, 10, e13779. [Google Scholar] [CrossRef]
- García-Grajales, J.; Buenrostro-Silva, A. Assessment of Human–Crocodile Conflict in Mexico: Patterns, Trends and Hotspots Areas. Mar. Freshw. Res. 2019, 70, 708. [Google Scholar] [CrossRef]
- González-Desales, G.; Sigler, L.; García-Grajales, J.; Charruau, P.; Zarco-González, M.; Monroy-vilchis, O.; Balbuena-Serrano, Á. Factors Influencing the Occurrence of Negative Interactions between People and Crocodilians in Mexico. Oryx 2021, 55, 791–799. [Google Scholar] [CrossRef]
- Brien, M.; Beri, P.; Coulson, S.; Frisby, T.; Perera, D.; Joyce, M. A Novel Method of Using a Drone to Capture Saltwater Crocodiles (Crocodylus porosus). Herpetol. Rev. 2020, 51, 32–37. [Google Scholar]



| Observed (Nu only) | Results from Regression | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Ni | Nu | HL (cm) | TL (cm) | Ratio TL/HL | Allometry Characteristics | RE | Variance Distribution by Sources of Imprecision | |||||||||||||||||
| Q1 | Med | Mean | Q3 | Min | Q1 | Med | Mean | Q3 | Max | Q1 | Med | Mean | Q3 | a | b | σ | R2 | (%) | HI (%) | HLM (%) | AV (%) | AR (%) | |||
| Alligator mississippiensis | 2391 | 2374 | 13.5 | 17.5 | 19.1 | 25.5 | 20.6 | 99.9 | 130.9 | 140.8 | 189.1 | 396.0 | 7.1 | 7.4 | 7.3 | 7.6 | 1.89 | 1.04 | 0.06 | 0.99 | 10.90 | 2.5 | 43.9 | 2.7 | 50.9 |
| Caiman crocodilus | 459 | 454 | 14.6 | 18.0 | 16.6 | 19.3 | 26.4 | 109.2 | 132.3 | 122.3 | 143.5 | 204.9 | 7.1 | 7.4 | 7.3 | 7.6 | 1.81 | 1.06 | 0.06 | 0.97 | 12.20 | 2.3 | 40.0 | 2.5 | 55.3 |
| Crocodylus acutus | 906 | 905 | 4.2 | 7.8 | 12.6 | 18.6 | 22.5 | 27.1 | 49.0 | 82.4 | 121.4 | 372.0 | 6.3 | 6.4 | 6.4 | 6.6 | 1.82 | 1.02 | 0.05 | 1.00 | 9.70 | 2.7 | 48.2 | 3.0 | 46.1 |
| Crocodylus intermedius | 403 | 396 | 5.7 | 7.3 | 8.3 | 9.3 | 23.7 | 35.2 | 46.8 | 51.2 | 56.5 | 197.0 | 6.0 | 6.1 | 6.3 | 6.4 | 1.84 | 1.00 | 0.08 | 0.96 | 14.80 | 1.6 | 28.5 | 1.6 | 68.2 |
| Crocodylus johnstoni | 588 | 539 | 4.0 | 4.6 | 7.2 | 9.6 | 18.6 | 25.9 | 29.4 | 42.4 | 55.0 | 230.2 | 6.1 | 6.4 | 6.4 | 6.7 | 2.02 | 0.91 | 0.06 | 0.98 | 13.00 | 1.7 | 29.9 | 1.5 | 67.0 |
| Crocodylus moreletii | 597 | 591 | 8.1 | 12.6 | 15.2 | 20.9 | 21.0 | 53.0 | 85.9 | 102.3 | 139.5 | 375.0 | 6.4 | 6.7 | 6.7 | 6.9 | 1.83 | 1.03 | 0.06 | 0.99 | 12.50 | 2.1 | 37.5 | 2.4 | 58.0 |
| Crocodylus niloticus | 340 | 340 | 4.2 | 9.6 | 19.0 | 38.7 | 27.2 | 32.0 | 71.2 | 136.6 | 275.1 | 413.6 | 7.1 | 7.3 | 7.3 | 7.6 | 2.01 | 0.99 | 0.07 | 1.00 | 13.50 | 1.8 | 32.3 | 1.9 | 64.0 |
| Crocodylus palustris | 80 | 79 | 21.0 | 31.0 | 34.3 | 47.8 | 43.0 | 144.5 | 196.5 | 206.5 | 260.3 | 487.0 | 5.5 | 6.5 | 6.4 | 7.2 | 2.49 | 0.81 | 0.12 | 0.94 | 24.30 | 0.5 | 8.9 | 0.4 | 90.2 |
| Crocodylus porosus | 370 | 368 | 6.4 | 8.2 | 10.5 | 11.5 | 26.9 | 41.5 | 54.3 | 71.3 | 77.9 | 332.5 | 6.5 | 6.6 | 6.7 | 6.8 | 1.78 | 1.05 | 0.04 | 0.99 | 8.40 | 3.2 | 55.7 | 3.3 | 37.9 |
| Crocodylus rhombifer | 196 | 193 | 15.5 | 22.8 | 25.0 | 34.0 | 94,0 | 109.8 | 163.0 | 176.5 | 234.0 | 330.0 | 6.9 | 7.1 | 7.1 | 7.2 | 2.05 | 0.97 | 0.05 | 0.98 | 10.10 | 2.5 | 43.7 | 2.2 | 51.6 |
| Crocodylus suchus | 116 | 115 | 7.0 | 10.3 | 13.9 | 18.4 | 34.2 | 49.8 | 69.1 | 94.8 | 126.0 | 250.0 | 6.7 | 6.9 | 6.9 | 7.1 | 2.02 | 0.96 | 0.05 | 0.99 | 9.40 | 2.7 | 46.9 | 2.7 | 47.8 |
| Gavialis gangeticus | 353 | 350 | 30.0 | 40.0 | 41.1 | 52.3 | 73.0 | 172.0 | 223.0 | 230.7 | 293.0 | 533.0 | 5.4 | 5.6 | 5.6 | 5.9 | 1.76 | 0.99 | 0.08 | 0.95 | 15.10 | 1.6 | 27.9 | 1.5 | 69.1 |
| Mecistops leptorhynchus | 159 | 159 | 8.6 | 12.2 | 17.0 | 21.1 | 33.8 | 50.7 | 70.1 | 96.1 | 120.4 | 302.0 | 5.6 | 5.8 | 5.7 | 5.9 | 1.83 | 0.97 | 0.03 | 1.00 | 5.80 | 3.8 | 66.6 | 3.4 | 26.1 |
| Melanosuchus niger | 167 | 167 | 9.2 | 15.1 | 17.1 | 23.8 | 31.2 | 73.5 | 121.1 | 131.9 | 188.7 | 283.5 | 7.5 | 7.7 | 7.7 | 8.0 | 2.02 | 1.01 | 0.06 | 0.99 | 11.20 | 2.4 | 41.0 | 2.4 | 54.2 |
| Osteolaemus tetraspis | 106 | 103 | 9.1 | 12.1 | 13.0 | 17.1 | 39.5 | 61.3 | 81.8 | 88.9 | 112.9 | 165.2 | 6.6 | 6.8 | 6.7 | 6.9 | 1.83 | 1.03 | 0.05 | 0.98 | 10.30 | 2.6 | 45.0 | 2.8 | 49.6 |
| Paleosuchus palpebrosus | 149 | 148 | 8.0 | 11.9 | 12.4 | 15.9 | 28.1 | 54.2 | 84.1 | 88.2 | 120.1 | 185.5 | 6.9 | 7.1 | 7.1 | 7.3 | 1.80 | 1.07 | 0.05 | 0.99 | 9.50 | 2.9 | 50.4 | 3.3 | 43.5 |
| Paleosuchus trigonatus | 87 | 87 | 12.9 | 15.7 | 16.2 | 19.5 | 50.0 | 81.3 | 102.8 | 103.8 | 127.7 | 183.0 | 6.2 | 6.4 | 6.4 | 6.6 | 1.65 | 1.08 | 0.04 | 0.98 | 7.70 | 3.4 | 58.5 | 3.3 | 34.8 |
| True Length (cm) | Altitude (m) | Average Estimation (cm) | Standard Deviation (cm) | Δ (cm) | Relative Error (%) |
|---|---|---|---|---|---|
| 13.7 | 20 | 14.02 | 0.58 | 0.32 | 2.4 |
| 13.7 | 30 | 14.30 | 0.82 | 0.60 | 4.4 |
| 13.7 | 40 | 14.22 | 0.72 | 0.52 | 3.8 |
| 27.3 | 20 | 27.70 | 0.71 | 0.40 | 1.4 |
| 27.3 | 30 | 27.99 | 0.92 | 0.69 | 2.5 |
| 27.3 | 40 | 27.95 | 0.82 | 0.65 | 2.4 |
| 40.8 | 20 | 41.09 | 0.51 | 0.29 | 0.7 |
| 40.8 | 30 | 41.75 | 1.10 | 0.95 | 2.3 |
| 40.8 | 40 | 41.80 | 0.67 | 1.00 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aubert, C.; Le Moguédec, G.; Velasco, A.; Combrink, X.; Lang, J.W.; Griffith, P.; Pacheco-Sierra, G.; Pérez, E.; Charruau, P.; Villamarín, F.; et al. Estimating Total Length of Partially Submerged Crocodylians from Drone Imagery. Drones 2024, 8, 115. https://doi.org/10.3390/drones8030115
Aubert C, Le Moguédec G, Velasco A, Combrink X, Lang JW, Griffith P, Pacheco-Sierra G, Pérez E, Charruau P, Villamarín F, et al. Estimating Total Length of Partially Submerged Crocodylians from Drone Imagery. Drones. 2024; 8(3):115. https://doi.org/10.3390/drones8030115
Chicago/Turabian StyleAubert, Clément, Gilles Le Moguédec, Alvaro Velasco, Xander Combrink, Jeffrey W. Lang, Phoebe Griffith, Gualberto Pacheco-Sierra, Etiam Pérez, Pierre Charruau, Francisco Villamarín, and et al. 2024. "Estimating Total Length of Partially Submerged Crocodylians from Drone Imagery" Drones 8, no. 3: 115. https://doi.org/10.3390/drones8030115
APA StyleAubert, C., Le Moguédec, G., Velasco, A., Combrink, X., Lang, J. W., Griffith, P., Pacheco-Sierra, G., Pérez, E., Charruau, P., Villamarín, F., Roberto, I. J., Marioni, B., Colbert, J. E., Mobaraki, A., Woodward, A. R., Somaweera, R., Tellez, M., Brien, M., & Shirley, M. H. (2024). Estimating Total Length of Partially Submerged Crocodylians from Drone Imagery. Drones, 8(3), 115. https://doi.org/10.3390/drones8030115






