Abstract
Climate change is exerting significant impacts on ecosystems worldwide, with alpine regions being particularly vulnerable. Alpine fauna is relatively poorly understood, particularly in terrain which is difficult for humans to survey. Knowledge of alpine species is further limited by a paucity of survey techniques that are widely applicable in this environment. Drones have potential as a low-impact tool for surveying fauna in remote alpine terrain. New Zealand’s diverse alpine lizards are an ideal system for exploring novel survey techniques. We build on previous research demonstrating the potential of drones for surveying alpine lizards by evaluating (1) how closely a drone can approach different alpine lizard species in scree, talus, and tussock-grassland habitats and (2) the effectiveness of drone surveys compared to traditional systematic visual searches for these species and habitats. The drone (model: DJI Mavic Air 2) was able to approach within 0.1–2.5 m of a lizard (mean = 0.77 m) before triggering a flight response. Systematic visual searches outperformed drone surveys in all habitats accessible to human observers. However, drones were relatively effective in talus habitats, demonstrating their potential utility in inaccessible rocky alpine habitats. Improvements to drone technology may further enhance the utility of drone-based surveys in ecological research.
Keywords:
drones; unmanned aerial vehicles; alpine; lizards; reptiles; drones; skinks; behaviour; flight response 1. Introduction
Conservation efforts and ecological research in remote or inaccessible terrain are currently hampered by a limited suite of effective survey and monitoring techniques [1,2]. Many of these ecosystems are highly sensitive to disturbance; thus, monitoring these sites can often be incredibly difficult [3]. Alpine habitats, forest canopies, high cliff faces, impenetrable vegetation, steep terrain, and remote landscapes are often the least understood terrestrial habitats. Challenges surrounding accessibility, ease of survey, and disturbance to fauna while attempting on-foot surveys contribute to this paucity of knowledge [3]. Consequently, conservation outcomes for native fauna found in these habitats are often limited due to our restricted knowledge of their basic ecology [2,4]. The development of new and effective methods for studying rare, cryptic, or poorly understood species is a high priority to facilitate improved ecological outcomes in habitats that are difficult to survey [5,6,7,8].
It is well-recognised that alpine research is currently limited by ineffective survey methods [9]. Traditional methods can cause disturbance to fragile or mobile alpine habitats [10], and the presence of human observers can disturb local fauna, influencing survey results [11,12,13]. Alpine regions are often comprised of large rocky areas (e.g., scree, talus, tors and boulderfields) interspersed with tussock grasslands, mosses, and small, prostrate shrubs and trees [14]. Methods must, therefore, be capable of surveying species in open, rough terrain with vast areas of refugia for wildlife. A variety of factors influence the effectiveness of each method, including weather, experience of the surveyor, species behaviour, and habitat type [9,15]. Additionally, alpine rockfields provide a three-dimensional habitat [16] in which animals can move relatively unconstrained beneath the surface but are also unstable and thus highly sensitive to disturbance by humans [10]. Alpine lizards commonly have small home ranges, frequently using the same refugia within their habitat [17,18,19,20,21]. Changes to the composition of these important refuges, for example, when turning rocks to find lizards [22], have detrimental effects on lizard communities, in particular on specialised species restricted to alpine environments [11,12,13]. Additionally, alpine lizards spend a greater amount of time basking to compensate for the lower temperatures associated with alpine environments [23] in order to maximise heat absorption and increase locomotive function.
Temperate lizards generally have very small home ranges, especially when they are at high density (e.g., 1.5 m2 for southern grass skinks in the Eglinton Valley, Fiordland, New Zealand; [24]). Their initial response to a visual predator is usually to freeze, relying on camouflage for protection, but lizards will flee if a potential predator makes a close approach to within ca. 3 m (e.g., [25]). Lizards that retreat from a potential predator will usually re-emerge within 5 min (all authors, pers. obs.). In cooler temperatures, lizards may be less reactive due to reduced locomotive function, whereas in higher temperatures, individuals are more active as they are nearer their optimum body temperatures [26]. Cooler individuals are also more likely to retreat into a refuge in comparison to warmer individuals, who often retreat across the surface (run away) when approached by a predator [26].
Historically, the most common methods for monitoring lizard species have been diurnal and nocturnal systematic searches (visual searches and hand searches), pitfall trapping, and funnel trapping [9,22,27,28,29,30]. Systematic searches are extensively used for herpetological work [9,31]. Visual searches perform well in areas with low vegetation cover and open habitats, whereas hand searches are most effective in locations with plentiful retreats that can be lifted, especially for finding nocturnal species during the day [32]. Neither form of systematic search can be used efficiently for species found within high canopy, very steep or impenetrable habitats, or for species that remain beneath the surface for significant periods of time [32]. Pitfall and funnel trapping are commonly used in habitats accessible to humans, but traps are difficult to transport into remote locations [32]. Bycatch is also an issue, with non-target species (e.g., small mammals and invertebrates) frequently caught within trapping devices [32]. All the currently used survey and monitoring techniques require access by humans. Within alpine ecosystems, habitats such as scree and talus rockfields often shift drastically when additional weight is placed atop the surface [10], thus altering the habitat composition of these regions and disturbing, or even accidentally killing, local fauna [11]. Novel research tools need to be developed to reduce the impact of human disturbance on these sensitive sites.
Aotearoa New Zealand is home to 125 native lizard taxa (skinks and geckos) [33] found in a variety of habitats, including alpine regions, forests, shrublands, wetlands and coastal areas [1]. At least 50 lizard species are found in the country’s alpine regions, 13 of which are considered obligate alpine species [34]. However, New Zealand’s lizards are poorly understood, with 53% of species being classified as “Data Poor” under the New Zealand Threat Classification System, meaning little is known about their basic ecology and distribution [31]. This is especially true for obligate alpine lizards, all of which are considered “Data Poor” due to a lack of survey techniques hampering knowledge acquisition [18,33].
The use of drones is becoming of increasing interest within the research community for undertaking field surveys, data collection, and wildlife monitoring [35]. Drones have also been used internationally in a wide variety of other studies to monitor bird nests, survey marine animal reproduction patterns, detect invasive lizard species, and gather data on species abundance, density, and distribution [5,6,8,25,36,37,38,39,40]. Other studies have used drones to map the habitat usage of species (spatial and temporal) [40,41], to assist in anti-poaching efforts and reduce human-animal conflict [42,43], and to monitor animal behaviour at both group- and individual-level along with the spatial organisation and migration of groups [40]. However, using drones to survey New Zealand lizard species is still relatively novel. A recent pilot study assessed the potential for drones as a survey and monitoring tool for lizard species in New Zealand and gained promising results [25]. A drone operator was able to find and photograph at close range both grand skinks (Oligosoma grande) and jewelled geckos (Naultinus gemmeus) in their natural habitat [25]. Jewelled geckos were able to be identified down to an individual level using dorsal photos taken during drone surveys, demonstrating the potential of this survey method [25].
Drones have potential as a new survey method for small lizards, especially in remote or inaccessible alpine terrain [3,25]. We expand on recent research by evaluating (1) species and habitat-specific differences in approach distance of the drone to alpine skink species before a flight response is triggered and (2) the performance of drone surveys relative to systematic visual searches for alpine skinks in different alpine habitat types.
2. Materials and Methods
Two survey methods were used to collect data: drone surveying and systematic visual searches. A DJI Mavic Air 2 (Shenzen DJI Sciences and Technologies Ltd., Shenzen, China) was used to conduct all drone-based surveying and to test drone approach distances to a lizard. Lachie Davidge (LD) was the pilot for all drone surveys and distance measures. The drone was flown in GPS mode to assist with stability during flight (it allowed the drone to hover with no assistance from the pilot). Surveys were restricted to fine conditions (warm, sunny, low wind) at key lizard-basking times in the mornings (9 a.m. to 12 p.m.) and evenings (4 p.m. to 7 p.m.) that were also suitable for flying the drone. Photos were taken from the drone of each lizard found where possible. Lizards were usually able to be identified at the species level during the drone flight. However, if identification was not possible during flight, then photos were used to determine species after survey completion. We included six heliothermic alpine skink species in the study (Table 1).

Table 1.
Descriptions of each skink species sampled during our study ordered based on snout-to-vent length (SVL). All species are diurnal. Conservation status as per Hitchmough, et al. [33] and habitat information from Purdie [44].
Fieldwork was conducted within Oteake Conservation Park, Central Otago, New Zealand and on private land near Little Mount Ida. Authority to work with these protected species was provided by the University of Otago’s Animal Ethics Committee (AUP-21-163), the local iwi, Ngāi Tahu, relevant landowners, and the Department of Conservation (DOC).
2.1. Drone Approach Distances
To better understand how alpine lizards will respond to the presence of a drone, a series of drone-to-lizard distance measures were conducted. Surveys were carried out in three different alpine habitats: scree-rockfields, talus-rockfields and tussock-grassland (Figure 1), to assess how close a drone can approach a lizard in different habitats. An observer would visually search a patch of desired habitat for lizards. Once an individual was found, the drone was launched approximately 5 m away from the skink. The pilot found the individual using the drone camera before an approach began. The drone flew approximately 1 m off the ground, and lizards were approached slowly to reduce disturbance. An estimation of distance was taken from the drone to the lizard’s location at which a flight response was induced or recorded as 0.1 m if a flight response was not triggered (as 0 m is not biologically realistic and was also not feasible for statistical analysis). Measures were recorded in metres to 0.1 m by an independent observer sitting perpendicularly between the drone and the lizard to gain an accurate view of the distance from the lizard to the drone. The observer sat approximately 3 to 5 m from the lizard to ensure an estimation of distance would be accurate and to reduce disturbance to the lizard. Photos of a lizard were taken by the drone operator at approximately 1 m intervals as the drone approached to identify the species.

Figure 1.
Examples of photos of several sites containing each of the desired habitat types: (a) scree-rockfield, (b) tussock-grassland, and (c) talus-rockfield.
We recorded species, habitat type (i.e., what habitat the individual was found: scree-rockfield, talus-rockfield and tussock-grassland), weather (temperature (°C), average wind speed (m/s) over 30 s and cloud cover (%)) and distance to drone at flight response (metres). The temperature was determined using a MedalistTM digital thermometer (Marayong, NSW, Australia) set in the shade at ground level. Average wind speed was measured using a Kestrel Waterproof Pocket Wind Meter (Kestrel–1000, Boothwyn, PA, USA) held into the wind at head height, and cloud cover was estimated as the percentage of cloud cover directly above the observer before the drone took flight.
2.2. Drone vs. Visual Search Comparison
Drone surveys and systematic visual searches were carried out to compare the performance of drone surveys to the current standard method for collecting data on alpine lizards. We made no assumptions about the detection probability of alpine skinks for either method, but recognise that detection probability is generally low for lizards. Surveys were conducted in 20 plots of 25 m × 25 m set in three habitat types (scree-rockfields, talus-rockfields, and tussock-grassland) to assess the effectiveness of each method in each alpine habitat. Plot sizes were measured using a Leupold RX-2800 TBR/W rangefinder (Beaverton, OR, USA), with the edges marked using fluorescent tape tied around a prominent rock or plant. Plots were surveyed multiple times via both methods to achieve 60 surveys of each type in total. A 15 min rest break was given to allow lizards disturbed during the previous survey time to re-emerge. Each survey was 12 min in length (approximately half of the drone battery life) to enable two drone surveys per full drone battery for logistical reasons. Refugia were not moved during systematic visual searches to avoid bias when compared to drone surveys. The method deployed first at each new plot was alternated. Plots were sufficiently distant from one another to reduce the disturbance of individuals in neighbouring plots and limit the potential for lizard movement between plots. Both drone surveys and systematic visual searches followed the same approximate path through the plot. When a lizard was found, the pilot/observer would pause to record data before continuing the search.
At the plot scale, we recorded survey type (drone or systematic visual search), plot number, number of lizards found per survey, and habitat type (i.e., what habitat the individual was found: scree-rockfield, talus-rockfield and tussock-grassland). At the individual skink level, we recorded the strength of the skink’s reaction to the disturbance (drone or human observer), if any (Table 2).

Table 2.
Definitions of reaction strength of each individual observed during fieldwork. Reaction strength was determined based on the strength of the reaction to the disturbance of the drone or visual observer (during systematic visual searches).
2.3. Statistical Analysis
Statistical analyses and subsequent graphs were conducted in R using the RStudio interface (Version 2023.06.0+421, RStudio Team, PBC, Boston, MA, USA).
2.3.1. Drone Approach Distances
We ran a Variance Inflation Factor (VIF) analysis to determine the strength of collinearity between each of the selected variables. Habitat type and species were highly correlated (VIF > 5); therefore, we did not use these variables together in the same model during analysis. We constructed 17 linear models (including a null model; R packages nlme, version 3.1-164; mgcv, version 1.9-0; lme4, version 1.1-35.1) with drone approach distance as the response variable and different combinations of each of the selected variables (temperature (°C), average wind speed (m/s) and cloud cover (%), species and habitat type). We used Akaike’s Information Criterion (AIC; R packages MuMin, version 1.47.5; MASS, version 7.3-60; car, version 3.1-2) model comparison to evaluate competing models. All lizards were sampled once to avoid repeated measures.
2.3.2. Drone vs. Visual Search Comparison
We used a Generalised Linear Mixed Effects Model (GLMM; R package glm2, version 1.2.1) to compare the performance of drone surveys to systematic visual searches. We used VIF to determine whether there was any collinearity between variables. All variables had a VIF score of less than 5, indicative of low levels of collinearity. We evaluated the effect of habitat type (scree-rockfield, talus-rockfield, and tussock-grassland), survey method (independent variables), and plot (grouping variable) on the number of lizards found per survey (counts, dependent variable). We used the AIC model comparison to evaluate competing models constructed using the dredge function in package MuMin (version 1.47.5).
3. Results
Fieldwork was undertaken between February and April 2023. Ambient temperature during fieldwork ranged between 5.7 and 27 °C, with a mean temperature of 15.5 °C. The average wind speed was between 0 and 4.9 m/s, with an overall average wind speed of 1.7 m/s. Cloud cover varied between 0 and 100%, with an average cloud cover of 24%.
3.1. Drone Approach Distances
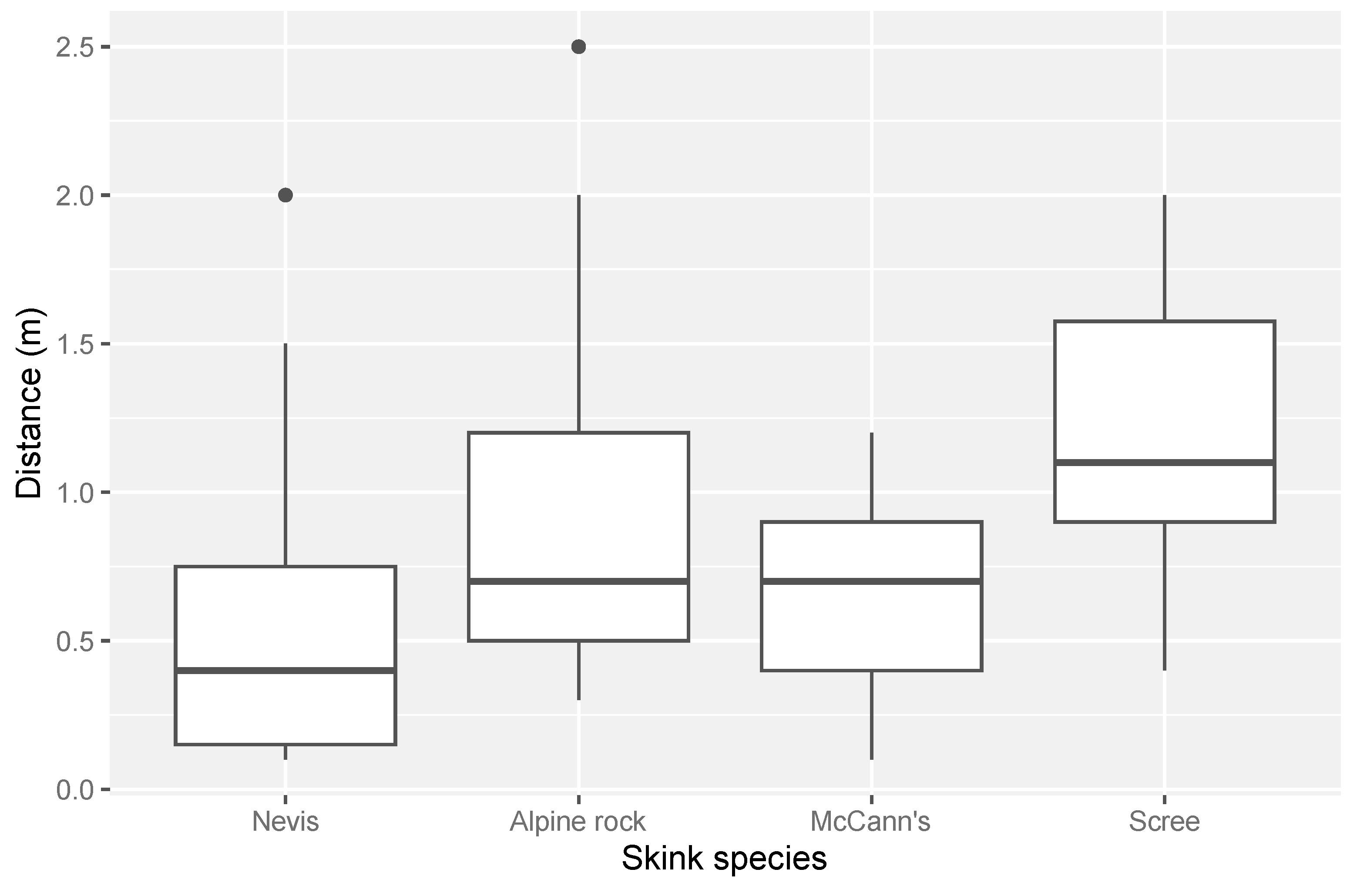
We conducted 88 distance measures in three habitat types (scree-rockfield, talus-rockfield, and tussock-grassland) and recorded the distance measurements of six different species of alpine skinks: Nevis skink (N = 31), alpine rock skink (N = 27), scree skink (N = 14), McCann’s skink (N = 13), southern grass skink (N = 2), and rockhopper skink (N = 1). Southern grass skinks and rockhopper skinks were omitted from statistical analysis due to low sample sizes. In total, 33 distance measures were taken in talus-rockfield-dominated habitat, 28 in tussock grassland, and 27 in scree-rockfield. Overall, drone approach distances ranged from 0.1 to 2.5 m across all species and habitats measured (mean = 0.77 m). The distance a drone could approach a lizard varied among species (Figure 2). Nevis skinks could be approached the closest on average, with a mean approach distance of 0.5 m (±0.1 m SE). In contrast, scree skinks were the most difficult to approach, with an average approach distance of 1.2 m (±0.2 m SE).
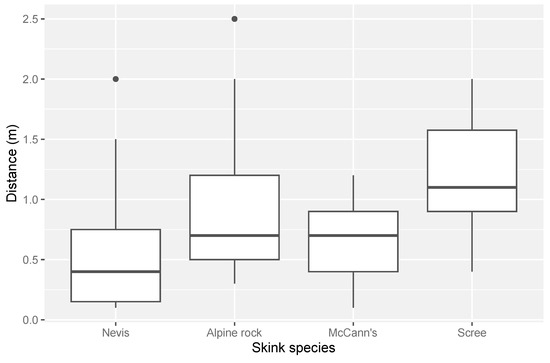
Figure 2.
The range of distances for each species to which a drone was able to approach an alpine skink before a flight response was induced. Sample sizes were alpine rock skink (N = 27), McCann’s skink (N = 13), Nevis skink (N = 31) and scree skink (N = 14).
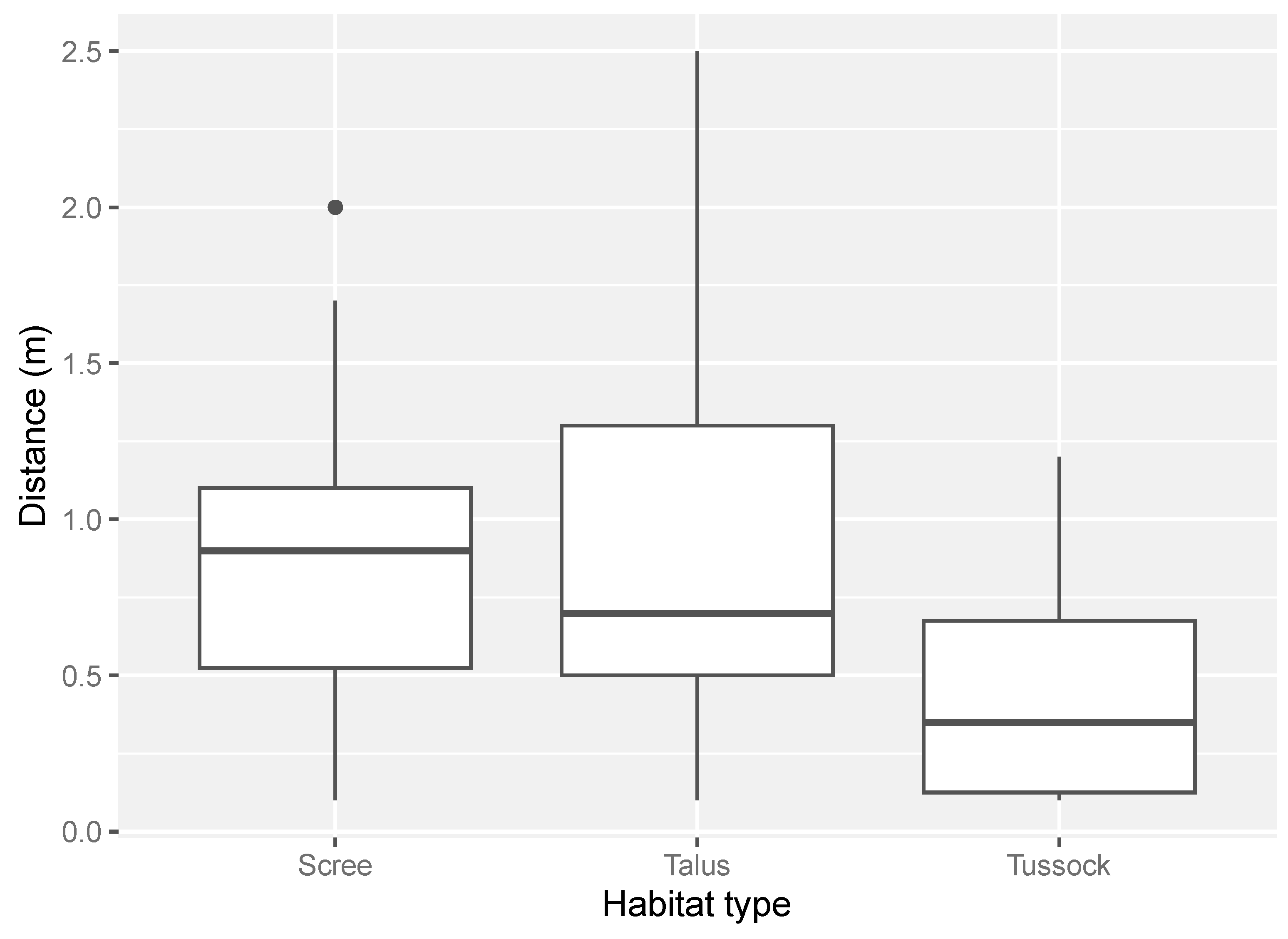
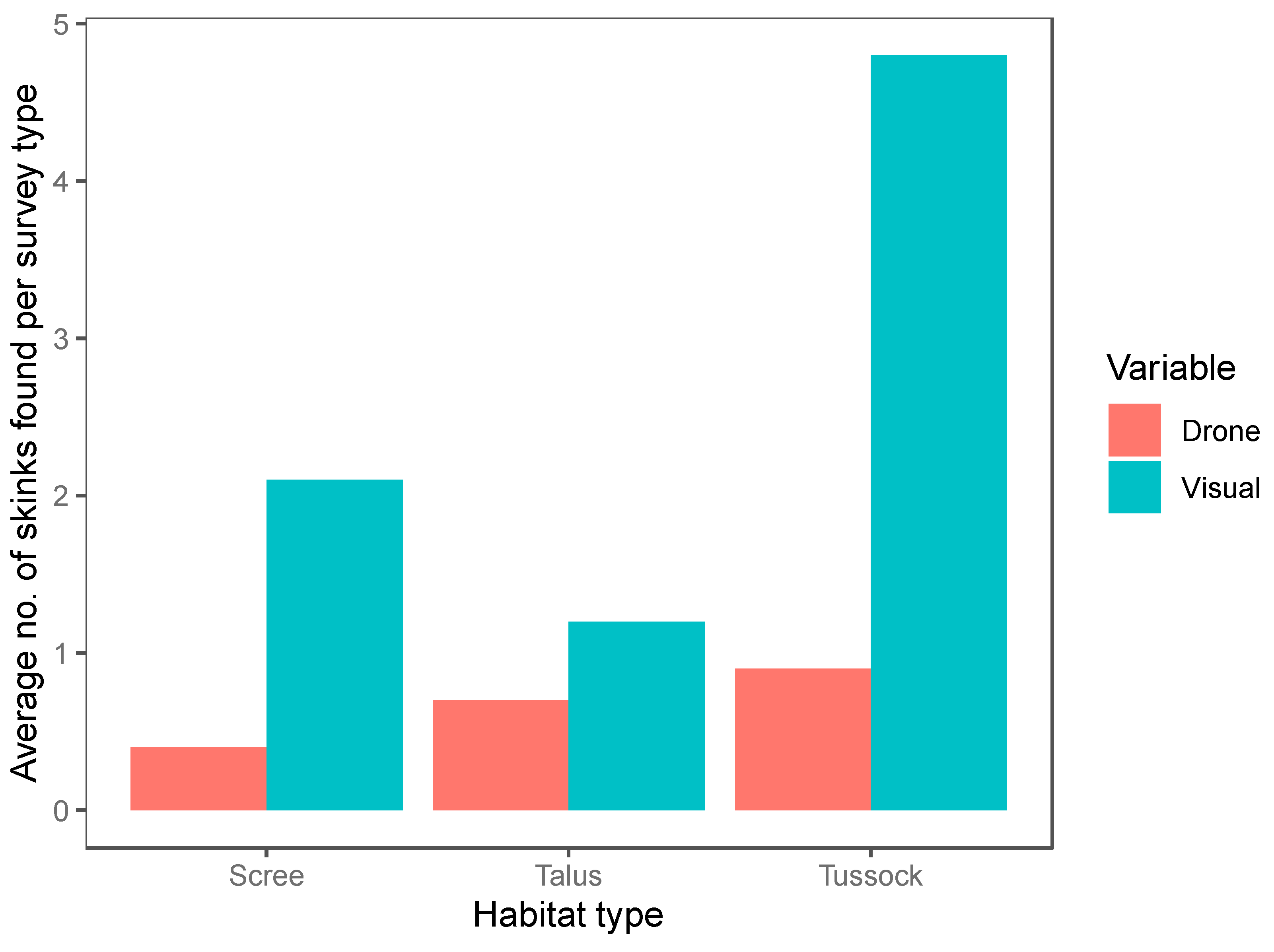
The distance to which a drone could approach a lizard varied among habitat types. Lizards found in scree and talus-rockfield were the most difficult to approach with the drone (mean in scree = 0.9 m ± 0.1 m SE; mean in talus = 0.9 m ± 0.1 m SE, Figure 3). In comparison, individuals in tussock grassland were the easiest to approach with a drone (mean = 0.5 m ± 0.1 m SE, Figure 3).
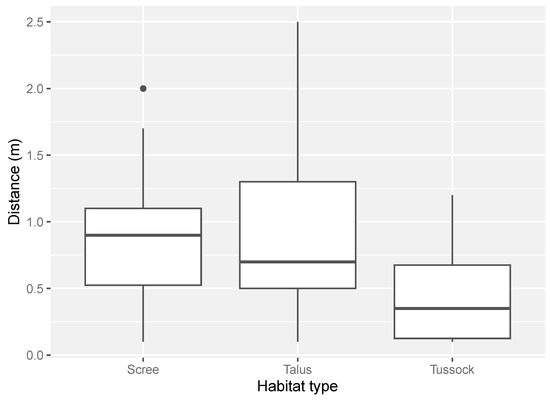
Figure 3.
The range of distances between a lizard and the drone in each habitat type surveyed. Distance was estimated in metres to one decimal place (scree = scree-rockfield, talus = talus-rockfield, tussock = tussock-grassland). The number of distance measures for each habitat type was: scree-rockfield (N = 27), talus-rockfield (N = 33) and tussock-grassland (N = 28).
Species, average wind speed, and temperature had the greatest effect on the distance a drone could approach a basking lizard, as evidenced by their inclusion in models with ΔAIC < 2 (Table 3). Skinks showed interspecific differences in tolerances to the presence of a drone (Figure 1). Further, the drone could approach skinks closer under lower average wind speeds and warmer temperatures. Habitat was also an important predictor, first appearing in a model with ΔAIC = 2.02 (Table 3; Figure 3). In contrast, the null model was not supported (ΔAIC = 18.6).

Table 3.
Distance to which a drone could approach an alpine skink species and relative influence of species, habitat type, temperature (temp), average wind speed (wind), and cloud cover (cloud). Species and habitats were not included in the same model as they were highly correlated to each other.
3.2. Drone vs. Visual Search Comparison
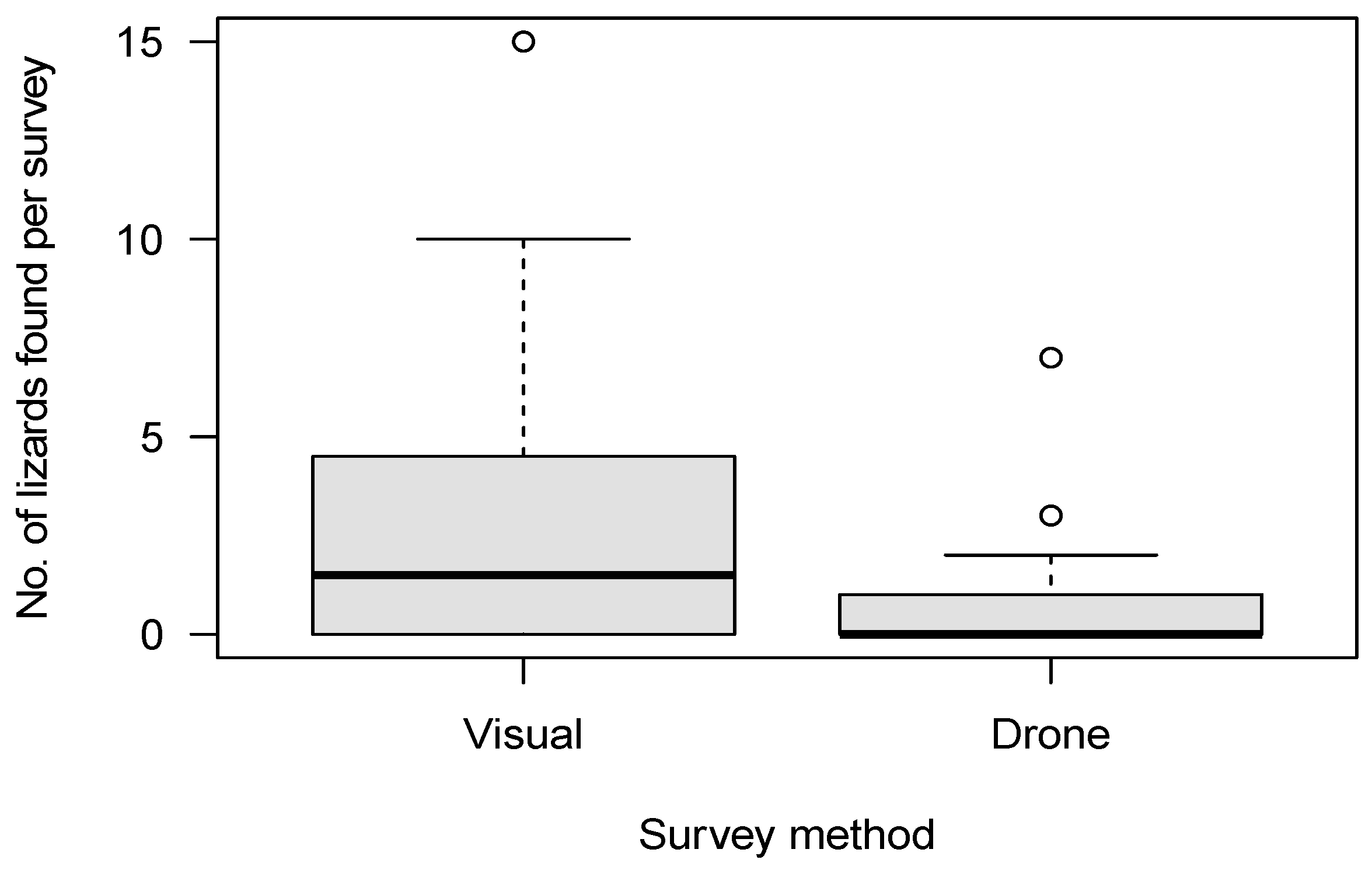
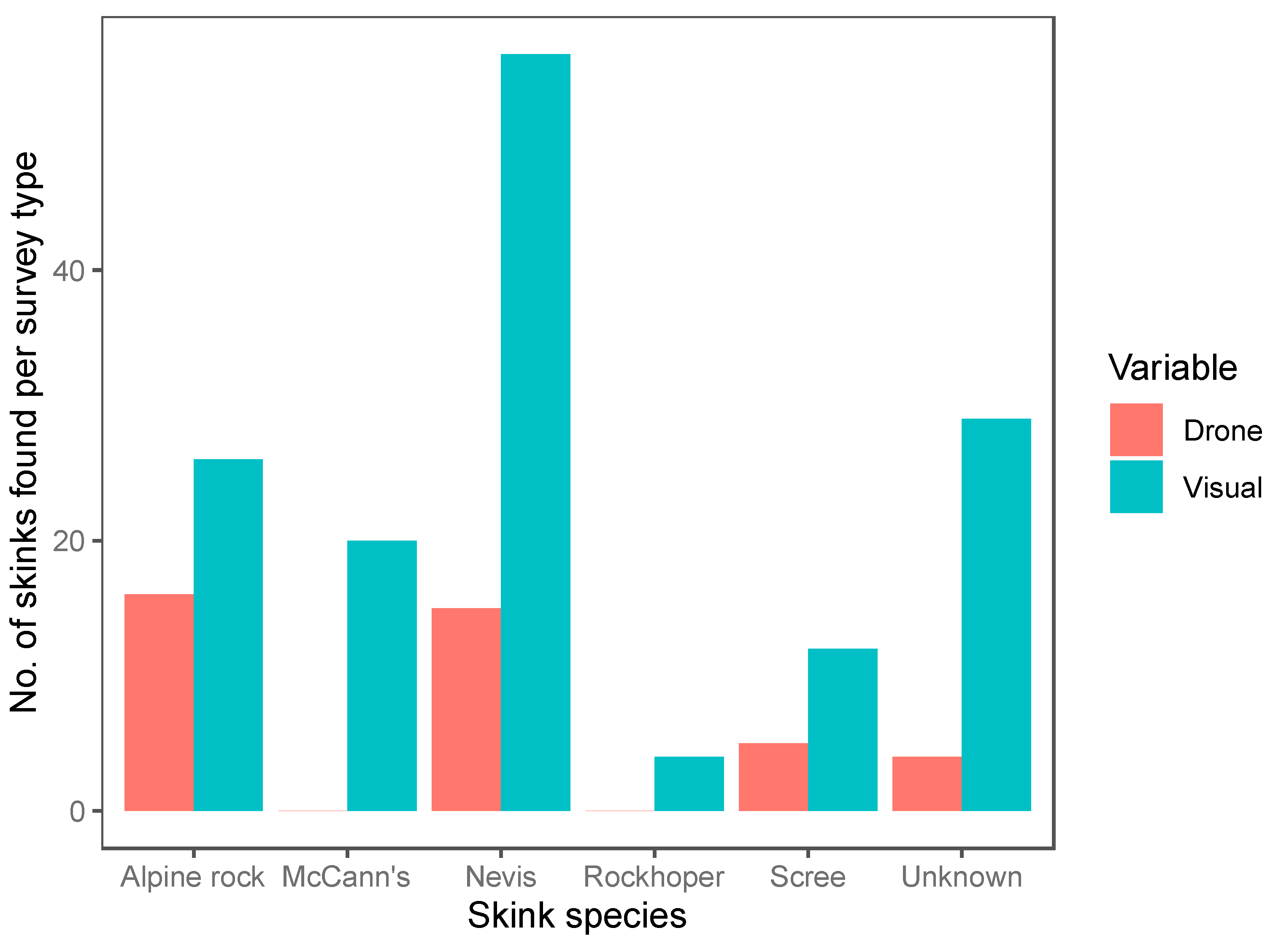
We conducted a total of 120 12-min surveys (60 systematic visual searches, 60 drone surveys) within 20 plots. Of the surveys, 42 were conducted in the tussock-grassland habitat, 48 in the talus-rockfield habitat and 30 in scree-rockfield. A total of 187 lizards were found during these surveys, encompassing 20 McCann’s skinks, 17 scree skinks, 42 alpine rock skinks, 4 rockhopper skinks, 71 Nevis skinks, and 33 unidentified skinks. Both the survey method and habitat type were important predictors of skink detection (Table 4). Overall, systematic visual searches were more successful at detecting skinks (N = 147) than drone surveys (N = 40; Figure 4) for all species (Figure 5). Using the drone survey method, we could only detect 27% of the skinks detected via systematic searching. Drone surveys were relatively successful in detecting alpine rock and scree skinks compared with other species (Figure 5 and Figure 6). Of the 60 drone surveys, one or more skinks were found in 24 (40%) of the surveys (mean = 0.66 ± 0.15 (SE) skinks per survey; range = 0 to 7; Figure 4). At least one skink was found on 39 (65%) of the systematic visual surveys (mean = 2.45 ± 0.38 SE skinks per survey; range = 0 to 15; Figure 4). Detection rates were highest in the tussock-grassland habitat (2.9 skinks per survey), especially for visual searches (4.8 skinks per survey; SE ± 0.9; range 0 to 15). Detections were substantially lower in both scree- and talus-rockfields (mean 1.3 and 0.9 per survey, respectively). Systematic visual searches outperformed drone surveys overall and in each habitat type (Figure 7). However, drone surveys performed relatively well in talus-rockfields (Figure 7).

Table 4.
Results from the AIC analysis assessing how the number of lizards observed per survey was influenced by habitat type and survey method (drone surveys vs. systematic visual searches).
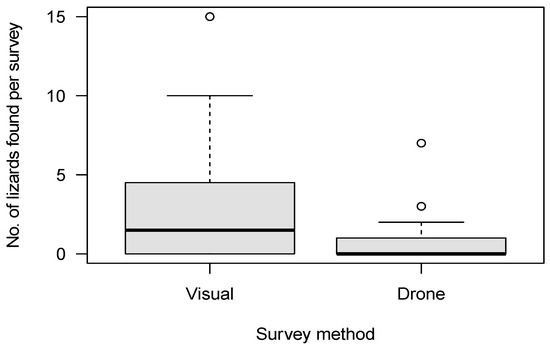
Figure 4.
The number of lizards found per 12 min survey for systematic visual searches (Visual) and drone surveys (Drone).
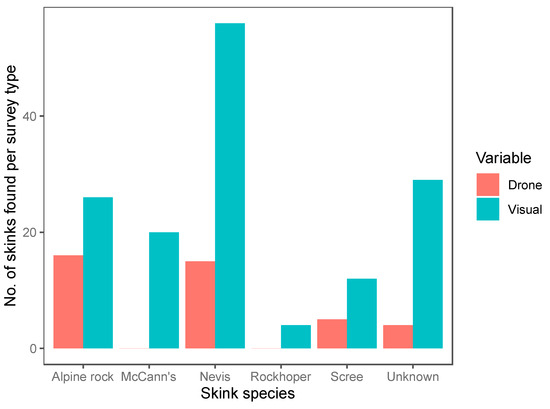
Figure 5.
The total number of individuals found per species for each survey method (drone surveys (Drone, red) and systematic visual searches (Visual, blue)). A total of 60 drone surveys and 60 systematic visual searches were undertaken during fieldwork.

Figure 6.
A drone photograph of a scree skink (visible in the centre, within the white circle) seen during a drone survey in a scree rockfield habitat.
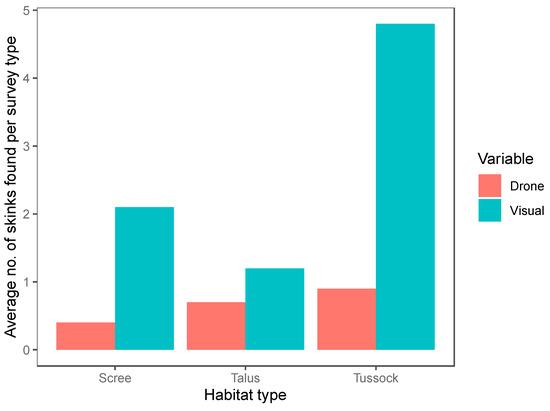
Figure 7.
The average number of skinks found per survey in each habitat type for drone surveys (red) and systematic visual searches (blue).
The reaction strength of skinks to disturbance was recorded for 183 (of the 187) skinks during method comparisons and was highly variable (Table 5). Overall, the strength of the reaction to the drone was lower than the reaction to human observers in visual surveys (Table 5). Overall, 37.5% of individuals showed no reaction to the drone, whereas only 18.9% of skinks showed no reaction to the human observer during systematic visual surveys (Table 5).

Table 5.
The reaction strength of skinks found during method comparison surveys for drone surveys (Drone, N = 40) and systematic visual searches (Visual, N = 143).
4. Discussion
We found that drones were able to approach most skinks closely (within 2.5 m). However, each species showed a different tolerance to the presence of a drone. Nevis skinks, primarily found in tussock grassland, were the most tolerant of the drone, allowing the pilot to position the drone directly above the individual in several instances. This lack of fear is likely due to reduced predator wariness caused by the higher vegetation cover within tussock grassland, with individuals being less conspicuous to predators [26]. In contrast, scree skinks, found most often within scree rockfields, were the least tolerant to the presence of the drone. Previous research has found that individuals basking in open areas show stronger responses to the approach of a predator (i.e., take flight sooner) than individuals in areas with higher vegetation density and more refuge sites [26,45]. Habitat type is likely a contributing factor influencing the tolerance of each skink species to the approach of the drone. Further, within a habitat type, the smaller-bodied species showed less wariness of the drone in comparison to larger-bodied species. This was expected, as body size influences the predation risk and refugia availability of lizards [26,46]. Larger-bodied skink species (e.g., alpine rock skinks and scree skinks) whose body size is similar to that of small predators (e.g., mice (Mus musculus) and weasels (Mustela erminea)) are at a higher risk of predation as these individuals have less access to refugia sites in which predators are unable to enter [46]. In comparison, smaller-bodied species (e.g., McCann’s skinks, Nevis skinks and rockhopper skinks) can escape into smaller crevices that these predators cannot enter [46,47]. Thus, habitat type, lizard body size, and refuge availability (and proximity) are all important factors influencing approach distance.
Overall, the drone surveys were outperformed by systematic visual searches in each habitat type surveyed. Drone surveys were limited by their field of vision and the pilot’s ability to detect motion on the small phone screen. In comparison, during systematic visual searches, the human observer was able to use their peripheral vision to detect movement far more easily and had a broader scale of vision, allowing them to see a greater proportion of the survey area at any one time. However, drone surveys performed relatively well in talus-rockfield. The open habitat of talus rockfields allowed the drone to move unhindered, and the low-lying/prostrate shrubs, grasses, and other alpine plants allowed the pilot a far better view of the landscape below. Consequently, finding basking lizards with a drone in these areas was relatively easy in comparison to more highly vegetated habitats. Alpine regions contain no tall growing trees and shrubs and often have vast swathes of open rockfields (i.e., scree and talus-rockfield, tors, cliffs) [14]. Drone surveys had no effect on the alpine habitats in which they were conducted as no human observer was required to move over the sensitive rockfield slopes. In these rocky areas, drones can fly uninhibited by vegetation and are thus able to find basking individuals relatively effectively. However, drone surveys within tussock grassland were vastly inferior to systematic visual searches. Air currents produced by drone flight caused swathes of vegetation to move in the wind. Flying above tussock grassland caused difficulties when attempting to locate lizards beneath the vegetation layer, further inhibiting the drone operator’s ability to detect already cryptic lizard species (LD pers. obs.).
During periods of warmer temperatures, the drone was able to approach lizards closer before they took flight. This is likely due to the increased locomotive ability of lizards in higher temperatures, with individuals able to react more quickly to a potential threat (i.e., drone) and, therefore, being less vulnerable to predation [23,48]. During windy weather, strong gusts disturbed drone flight and caused the camera to become unstable, reducing the pilot’s ability to detect small skinks. Further, sudden changes in the drone’s movement generated by wind gusts caused the skinks to retreat more often in the drone’s presence, reducing the drone pilot’s ability to find and identify individuals (LD pers. obs.).
To our knowledge, ours is the first study to investigate the disturbance to lizards from a drone in comparison with a human observer. Systematic visual searches disturbed a greater proportion of lizards than drone searches, resulting in many individuals being spotted as they retreated from the observer (Table 5). In comparison, drone surveys caused far less disturbance, with 37.5% of skink showing no reaction to the presence of a drone. Despite some studies cautioning against the use of drones in animal research, e.g., [49,50], our results have demonstrated the potential of drones in ecological research, with New Zealand alpine skink species showing higher tolerance to drones in comparison to systematic visual searches. However, it is important to consider species-level differences in tolerance to drones [51,52]. Improvements in drone technology, such as reduced noise, higher camera quality, and a camera zoom function, may further reduce drone disturbance and allow researchers to gain valuable ecological data in remote or inaccessible areas to human operators [25,52].
Due to the limited photo quality of the drone used during our study, skinks could not always be identified at the species level using photo identification. Many skink species are small and have evolved effective camouflage to combat avian predation [18,44]. Further, it can be challenging to differentiate among species of New Zealand’s morphologically cryptic skinks; differences in the number of supraocular scales, body shape, and scale patterning are commonly used to differentiate among species [18,44,53]. In contrast, many arboreal gecko species can be identified at the individual level using differences in dorsal patterns [53]. For these species, the use of drones may allow us to identify individuals using novel drone technology [25].
Our research has shown that drone surveys perform relatively well in open, rocky habitats (especially talus rockfields) and will provide new opportunities for studying lizard species in remote or inaccessible alpine terrain. Although weather influences drone survey performance, lizard activity is also positively associated with fine weather (i.e., low wind and warm). As such, the influence of weather on survey results can be mitigated by carefully choosing appropriate weather for both drone flying and lizard surveying. Based on our findings, drone surveys for alpine lizards of the temperate zone are most effective in relatively warm temperatures (ca. 15 to 27 °C), in low levels of wind (ca. 0 to 2 m/s), and in open rocky habitats. Our field experience also suggests that it is important for drone pilots to put effort into reducing unnecessary movement whilst approaching a lizard to reduce potential disturbance, such as flying slowly along a single path and low to the ground to enhance the detection of lizards before they take flight.
Overall, drones have the potential to expand our understanding of cryptic alpine lizard species in remote or inaccessible alpine terrain, which is likely to be enhanced by future improvements to drone technology. Drone technology also has the potential to reduce human disturbance in sensitive alpine ecosystems, improve our knowledge of rare or endangered alpine lizard species, and improve access to remote or inaccessible terrain. It is, therefore, imperative that continued effort is put into further developing new, effective tools for monitoring remotely located alpine species. Drones have the potential to provide data on habitat associations of lizards in inaccessible terrain and to measure population trends over time that will ultimately better direct conservation efforts [1,9,15,17,54].
Author Contributions
Conceptualization: C.D.K. and J.M.M.; Methodology: J.M.M., C.D.K. and L.R.D.; Formal analysis: L.R.D. and J.M.M.; Investigation: L.R.D.; Resources: L.R.D. and J.M.M.; Data curation: L.R.D.; Writing—original draft preparation: L.R.D.; Writing—review and editing: J.M.M. and C.D.K.; Visualization: L.R.D. and J.M.M.; Supervision: J.M.M. and C.D.K.; Project administration: J.M.M. and L.R.D.; Funding acquisition: J.M.M. and L.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the University of Otago’s Animal Ethics Committee (AUP-21-163, 20 October 2022). Approval was also granted to by the Department of Conservation Lizard Technical Advisory Group (DOC – 7215029).
Data Availability Statement
Data on fieldwork locations are not available for species protection reason (i.e., poaching risk). Other data are available upon reasonable request to the corresponding author.
Acknowledgments
A huge thank you to Raphael Davidge, Ngawhira Kennedy, Harriet Wills and Pearl Barry for their outstanding assistance during fieldwork. Further, thanks go out to Aaron Bertoia for your assistance with statistical analysis and to the RAWE research group for providing invaluable feedback on the draft manuscript during the review period. A final thank you to the landowners who gave us permission to conduct fieldwork on their property.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hitchmough, R.; Adams, L.; Reardon, J.; Monks, J. Current challenges and future directions in lizard conservation in New Zealand. J. R. Soc. N. Z. 2016, 46, 29–39. [Google Scholar] [CrossRef]
- Monks, J.M.; O’Donnell, C.F.; Greene, T.C.; Weston, K.A. Evaluation of counting methods for monitoring populations of a cryptic alpine passerine, the rock wren (Passeriformes, Acanthisittidae, Xenicus gilviventris). PLoS ONE 2021, 16, e0247873. [Google Scholar] [CrossRef]
- Bollard, B.; Doshi, A.; Gilbert, N.; Poirot, C.; Gillman, L. Drone technology for monitoring protected areas in remote and fragile environments. Drones 2022, 6, 42. [Google Scholar] [CrossRef]
- Fettermann, T.; Fiori, L.; Gillman, L.; Stockin, K.A.; Bollard, B. Drone surveys are more accurate than boat-based surveys of bottlenose dolphins (Tursiops truncatus). Drones 2022, 6, 82. [Google Scholar] [CrossRef]
- Schofield, G.; Katselidis, K.A.; Lilley, M.K.; Reina, R.D.; Hays, G.C. Detecting elusive aspects of wildlife ecology using drones: New insights on the mating dynamics and operational sex ratios of sea turtles. Funct. Ecol. 2017, 31, 2310–2319. [Google Scholar] [CrossRef]
- Dubos, N.; Porcel, X.; Roesch, M.; Claudin, J.; Pinel, R.; Probst, J.-M.; Deso, G. A bird’s-eye view: Evaluating drone imagery for the detection and monitoring of endangered and invasive day gecko species. Biotropica 2023, 55, 911–919. [Google Scholar] [CrossRef]
- Weimerskirch, H.; Prudor, A.; Schull, Q. Flights of drones over sub-Antarctic seabirds show species-and status-specific behavioural and physiological responses. Polar Biol. 2018, 41, 259–266. [Google Scholar] [CrossRef]
- Aota, T.; Ashizawa, K.; Mori, H.; Toda, M.; Chiba, S. Detection of Anolis carolinensis using drone images and a deep neural network: An effective tool for controlling invasive species. Biol. Invasions 2021, 23, 1321–1327. [Google Scholar] [CrossRef]
- Lettink, M.; Monks, J. Survey and monitoring methods for New Zealand lizards. J. R. Soc. N. Z. 2016, 46, 16–28. [Google Scholar] [CrossRef]
- Bell, K.L.; Bliss, L.C. Alpine disturbance studies: Olympic National Park, USA. Biol. Conserv. 1973, 5, 25–32. [Google Scholar] [CrossRef]
- Sato, C.; Wood, J.T.; Schroder, M.; Green, K.; Michael, D.; Lindenmayer, D.B. The impacts of ski resorts on reptiles: A natural experiment. Anim. Conserv. 2014, 17, 313–322. [Google Scholar] [CrossRef]
- D’cruze, N.; Kumar, S. Effects of anthropogenic activities on lizard communities in northern Madagascar. Anim. Conserv. 2011, 14, 542–552. [Google Scholar] [CrossRef]
- Hecnar, S.J. Effects of human disturbance on five-lined skink, Eumeces fasciatus, abundance and distribution. Biol. Conserv. 1998, 85, 213–222. [Google Scholar] [CrossRef]
- Mark, A.F.; Galloway, D.J. Above the Treeline: A Nature Guide to Alpine New Zealand; Craig Potton Publishing: Nelson, New Zealand, 2012. [Google Scholar]
- Lettink, M.; Hare, K.M. Sampling techniques for New Zealand lizards. In New Zealand Lizards; Springer International Publishing: Cham, Switzerland, 2016; pp. 269–291. [Google Scholar]
- Love, D. Subarctic and subalpine: Where and what? Arct. Alp. Res. 1970, 2, 63–73. [Google Scholar] [CrossRef]
- Hare, K.M.; Chapple, D.G.; Towns, D.R.; van Winkel, D. The ecology of New Zealand’s lizards. In New Zealand Lizards, Chapple, D.G., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 133–168. [Google Scholar]
- van Winkel, D.; Hitchmough, R.; Baling, M. Reptiles and Amphibians of New Zealand: A Field Guide; Auckland University Press: Auckland, New Zealand, 2018. [Google Scholar]
- McIntyre, N.E.; Wiens, J.A. Interactions between landscape structure and animal behavior: The roles of heterogeneously distributed resources and food deprivation on movement patterns. Landsc. Ecol. 1999, 14, 437–447. [Google Scholar] [CrossRef]
- Neilson, K.; Curran, J.M.; Towns, D.R.; Jamieson, H. Habitat use by chevron skinks (Oligosoma homalonotum)(Sauria: Scincidae) on Great Barrier Island, New Zealand. N. Z. J. Ecol. 2006, 30, 345–356. [Google Scholar]
- Plummer, M.V.; Mills, N.E. Spatial ecology and survivorship of resident and translocated hognose snakes (Heterodon platirhinos). J. Herpetol. 2000, 34, 565–575. [Google Scholar] [CrossRef]
- Knox, C.D.; Jewell, T.R.; Monks, J.M. Ecology of orange-spotted geckos (Mokopirirakau “Roys Peak”) in Central Otago and Queenstown-lakes districts. N. Z. J. Ecol. 2019, 43, 1–9. [Google Scholar] [CrossRef]
- Bertoia, A.; Monks, J.; Knox, C.; Cree, A. A nocturnally foraging gecko of the high-latitude alpine zone: Extreme tolerance of cold nights, with cryptic basking by day. J. Therm. Biol. 2021, 99, 102957. [Google Scholar] [CrossRef] [PubMed]
- Lettink, M.; O’Donnell, C.F.; Hoare, J.M. Accuracy and precision of skink counts from artificial retreats. N. Z. J. Ecol. 2011, 35, 236–246. [Google Scholar]
- Monks, J.M.; Wills, H.P.; Knox, C.D. Testing drones as a tool for surveying lizards. Drones 2022, 6, 199. [Google Scholar] [CrossRef]
- Cooper, W., Jr. Risk factors affecting escape behavior by the desert iguana, Dipsosaurus dorsalis: Speed and directness of predator approach, degree of cover, direction of turning by a predator, and temperature. Can. J. Zool. 2003, 81, 979–984. [Google Scholar] [CrossRef]
- Bell, T.P.; Patterson, G.B. A rare alpine skink Oligosoma pikitanga n. sp.(Reptilia: Scincidae) from Llawrenny Peaks, Fiordland, New Zealand. Zootaxa 2008, 1882, 57–68. [Google Scholar] [CrossRef]
- Jewell, T.R.; Leschen, R.A. A new species of Hoplodactylus (Reptilia: Pygopodidae) from the Takitimu Mountains, South Island, New Zealand. Zootaxa 2004, 792, 1–11. [Google Scholar] [CrossRef]
- Patterson, G.B.; Bell, T.P. The Barrier skink Oligosoma judgei n. sp. (Reptilia: Scincidae) from the Darran and Takitimu Mountains, South Island, New Zealand. Zootaxa 2009, 2271, 43–56. [Google Scholar] [CrossRef]
- Whitaker, A. Hoplodactylus kahutarae n. sp. (Reptilia: Gekkonidae) from the seaward Kaikoura Range, Marlborough, New Zealand. N. Z. J. Zool. 1984, 11, 259–270. [Google Scholar] [CrossRef]
- Sewell, D.; Guillera-Arroita, G.; Griffiths, R.A.; Beebee, T.J. When is a species declining? Optimizing survey effort to detect population changes in reptiles. PLoS ONE 2012, 7, e43387. [Google Scholar] [CrossRef]
- Hare, K.M. Herpetofauna Model of the DOC Inventory and Monitoring Toolbox; Department of Conservation: Wellington, New Zealand, 2012. [Google Scholar]
- Hitchmough, R.; Barr, B.; Knox, C.; Lettink, M.; Monks, J.; Patterson, G.; Reardon, J.; van Winkel, D.; Rolfe, J.; Michel, P. Conservation Status of New Zealand Reptile, 2021; New Zealand Threat Classification Series 35; Department of Conservation: Wellington, New Zealand, 2021. [Google Scholar]
- O’Donnell, C.F.; Weston, K.A.; Monks, J.M. Impacts of introduced mammalian predators on New Zealand’s alpine fauna. N. Z. J. Ecol. 2017, 41, 1–22. [Google Scholar] [CrossRef]
- Chabot, D.; Bird, D.M. Wildlife research and management methods in the 21st century: Where do unmanned aircraft fit in? J. Unmanned Veh. Syst. 2015, 3, 137–155. [Google Scholar] [CrossRef]
- Sarda-Palomera, F.; Bota, G.; Viñolo, C.; Pallarés, O.; Sazatornil, V.; Brotons, L.; Gomáriz, S.; Sardà, F. Fine-scale bird monitoring from light unmanned aircraft systems. Ibis 2012, 154, 177–183. [Google Scholar] [CrossRef]
- Schofield, G.; Esteban, N.; Katselidis, K.A.; Hays, G.C. Drones for research on sea turtles and other marine vertebrates—A review. Biol. Conserv. 2019, 238, 108214. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; McLean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]
- Hensel, E.; Wenclawski, S.; Layman, C.A. Using a small, consumer-grade drone to identify and count marine megafauna in shallow habitats. Lat. Am. J. Aquat. Res. 2018, 46, 1025–1033. [Google Scholar] [CrossRef]
- Torney, C.J.; Lamont, M.; Debell, L.; Angohiatok, R.J.; Leclerc, L.-M.; Berdahl, A.M. Inferring the rules of social interaction in migrating caribou. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170385. [Google Scholar] [CrossRef]
- Strandburg-Peshkin, A.; Farine, D.R.; Crofoot, M.C.; Couzin, I.D. Habitat and social factors shape individual decisions and emergent group structure during baboon collective movement. eLife 2017, 6, e19505. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.R.; Gibbs, L.; Knott, N.A.; Broad, A.; Hing, M.; Taylor, M.D.; Davis, A.R. Coexisting with sharks: A novel, socially acceptable and non-lethal shark mitigation approach. Sci. Rep. 2020, 10, 17497. [Google Scholar] [CrossRef]
- Jiménez López, J.; Mulero-Pázmány, M. Drones for conservation in protected areas: Present and future. Drones 2019, 3, 10. [Google Scholar] [CrossRef]
- Purdie, S. A Naturalist’s Guide to the Reptiles & Amphibians of New Zealand; Mayer, K., Ed.; John Beaufoy Publishing Limited: Oxford, UK, 2022; p. 176. [Google Scholar]
- Zani, P.; Jones, T.; Neuhaus, R.; Milgrom, J. Effect of refuge distance on escape behavior of side-blotched lizards (Uta stansburiana). Can. J. Zool. 2009, 87, 407–414. [Google Scholar] [CrossRef]
- Tingley, R.; Hitchmough, R.A.; Chapple, D.G. Life-history traits and extrinsic threats determine extinction risk in New Zealand lizards. Biol. Conserv. 2013, 165, 62–68. [Google Scholar] [CrossRef]
- Whitaker, A. Lizard populations on islands with and without Polynesian rats, Rattus exulans (Peale). Proc. (N. Z. Ecol. Soc.) 1973, 20, 121–130. [Google Scholar]
- Rand, A.S. Inverse relationship between temperature and shyness in the lizard Anolis lineatopus. Ecology 1964, 45, 863–864. [Google Scholar] [CrossRef]
- Bennitt, E.; Bartlam-Brooks, H.L.; Hubel, T.Y.; Wilson, A.M. Terrestrial mammalian wildlife responses to unmanned aerial systems approaches. Sci. Rep. 2019, 9, 2142. [Google Scholar] [CrossRef] [PubMed]
- Ditmer, M.A.; Vincent, J.B.; Werden, L.K.; Tanner, J.C.; Laske, T.G.; Iaizzo, P.A.; Garshelis, D.L.; Fieberg, J.R. Bears show a physiological but limited behavioral response to unmanned aerial vehicles. Curr. Biol. 2015, 25, 2278–2283. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.R.; Green, C.M.; DeMaso, S.J.; Hardy, T.B. Drone surveys do not increase colony-wide flight behaviour at waterbird nesting sites, but sensitivity varies among species. Sci. Rep. 2020, 10, 3781. [Google Scholar] [CrossRef]
- Li, X.; Huang, H.; Savkin, A.V. Autonomous navigation of an aerial drone to observe a group of wild animals with reduced visual disturbance. IEEE Syst. J. 2022, 16, 3339–3348. [Google Scholar] [CrossRef]
- Knox, C.D.; Cree, A.; Seddon, P.J. Accurate identification of individual geckos (Naultinus gemmeus) through dorsal pattern differentiation. N. Z. J. Ecol. 2013, 37, 60–66. [Google Scholar]
- Chapple, D.G.; Hitchmough, R.A. Biogeography of New Zealand lizards. In New Zealand Lizards; Springer International Publishing: Cham, Switzerland, 2016; pp. 109–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).