Fabrication of Demineralized Bone Matrix/Polycaprolactone Composites Using Large Area Projection Sintering (LAPS)
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. DBM/PCL Ratio Selection
2.3. Additive Manufacturing
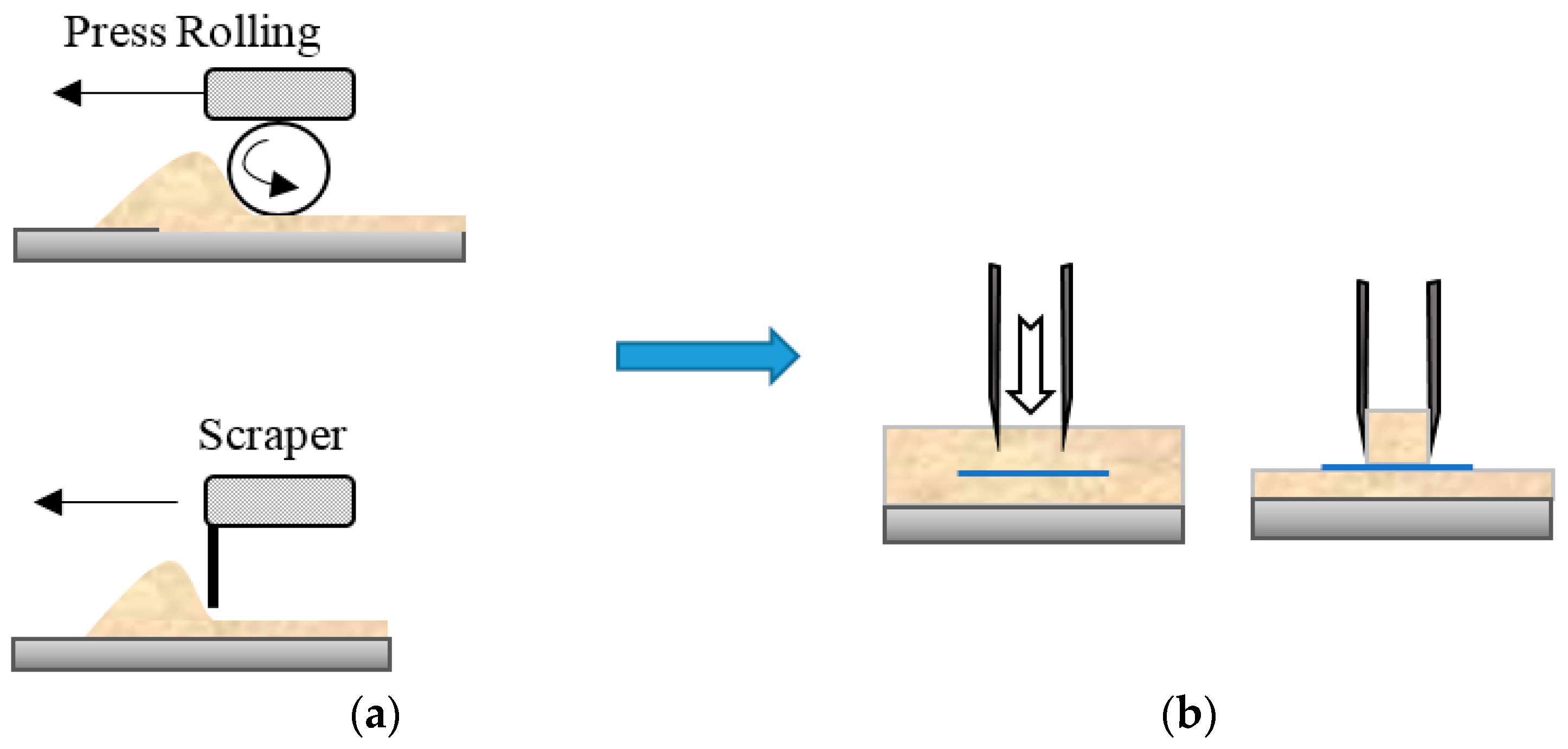
Powder Bed Formation
2.4. Reference Controls
2.5. Sample Preparation for Tensile Testing
3. Results and Discussion
3.1. Optimal Conditions for Bed Formation
3.2. Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, A.A.; Tsang, V.L.; Albrecht, D.R.; Bhatia, S.N. 3-D Fabrication Technology for Tissue Engineering. In BioMEMS and Biomedical Nanotechnology: Volume III Therapeutic Micro/Nanotechnology; Ferrari, M., Desai, T., Bhatia, S., Eds.; Springer: Boston, MA, USA, 2007; pp. 23–38. [Google Scholar]
- Sultana, N.; Wang, M. Fabrication of Tissue Engineering Scaffolds Using the Emulsion Freezing/Freeze-drying Technique and Characteristics of the Scaffolds. In Integrated Biomaterials in Tissue Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 63–89. [Google Scholar]
- Sin, D.; Miao, X.; Liu, G.; Wei, F.; Chadwick, G.; Yan, C.; Friis, T. Polyurethane (PU) scaffolds prepared by solvent casting/particulate leaching (SCPL) combined with centrifugation. Mater. Sci. Eng. C 2010, 30, 78–85. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Hatami, J.; Lehner, A.N.; da Silva, C.L.; Ferreira, F.C.; Antunes, A.P.M. Gelatin porous scaffolds fabricated using a modified gas foaming technique: Characterisation and cytotoxicity assessment. Mater. Sci. Eng. C 2015, 48, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Grinberg, A.; Gil, E.S.; Panilaitis, B.; Kaplan, D.L. High-strength silk protein scaffolds for bone repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef]
- Tada, S.; Stegaroiu, R.; Kitamura, E.; Miyakawa, O.; Kusakari, H. Influence of implant design and bone quality on stress/strain distribution in bone around implants: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implants 2003, 18, 357–368. [Google Scholar]
- Chumnanklang, R.; Panyathanmaporn, T.; Sitthiseripratip, K.; Suwanprateeb, J. 3D printing of hydroxyapatite: Effect of binder concentration in pre-coated particle on part strength. Mater. Sci. Eng. C 2007, 27, 914–921. [Google Scholar] [CrossRef]
- Liu, F.-H.; Lee, R.-T.; Lin, W.-H.; Liao, Y.-S. Selective laser sintering of bio-metal scaffold. In Proceedings of the 1st CIRP Conference on BioManufacturing, BioM 2013, Tokyo, Japan, 3–5 March 2013; pp. 83–87. [Google Scholar]
- Liulan, L.; Aili, T.; Huicun, Z.; Qingxi, H.; Minglun, F. The mechanical properties of bone tissue engineering scaffold fabricating via selective laser sintering. In International Conference on Life System Modeling and Simulation; LSMS 2007; Springer: Berlin/Heidelberg, Germany, 2007; pp. 146–152. [Google Scholar]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J. Biomed Mater. Res. B 2003, 64B, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Asaoka, T.; Furukawa, K.; Ushida, T. Fabrication of scaffold for bone regeneration by taylor made stereolithography. Adv. Sci. Technol. 2013, 86, 70–74. [Google Scholar] [CrossRef]
- Cuidi, L.; Li, G.; Fangping, C.; Changsheng, L. Fabrication of mesoporous calcium silicate/calcium phosphate cement scaffolds with high mechanical strength by freeform fabrication system with micro-droplet jetting. J. Mater. Sci. 2015, 50, 7182–7191. [Google Scholar]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Ju-Yeon, L.; Bogyu, C.; Wu, B.; Min, L. Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 2013, 5, 045003. [Google Scholar]
- Lin, L.; Rong, B.; Hu, Q.; Fang, M. Microstructural Comparison and Analysis of Bone Scaffold Prepared by FDM and SLS Process. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 109–112. [Google Scholar]
- Sa, M.W.; Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef]
- Yen, H.-J.; Tseng, C.-S.; Hsu, S.-H.; Tsai, C.-L. Evaluation of chondrocyte growth in the highly porous scaffolds made by fused deposition manufacturing (FDM) filled with type II collagen. Biomed. Microdevices 2009, 11, 615–624. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- La, W.-G.; Jang, J.; Kim, B.S.; Lee, M.S.; Cho, D.-W.; Yang, H.S. Systemically replicated organic and inorganic bony microenvironment for new bone formation generated by a 3D printing technology. RSC Adv. 2016, 6, 11546–11553. [Google Scholar] [CrossRef]
- Moloye, T.; Batich, C. Preparation and in vitro characterization of polycaprolactone and demineralized bone matrix scaffolds. In Proceedings of the 2011 MRS Fall Meeting, Boston, MA, USA, 28 November–2 December 2011; pp. 23–28. [Google Scholar]
- Singh, S.; Ramakrishna, S. Biomedical Applications of Additive Manufacturing: Present and Future. Curr. Opin. Biomed. Eng. 2017, 2, 105–115. [Google Scholar] [CrossRef]
- Nussbaum, G.C.J.; Harmon, J.; Crane, N. Evaluation of the Processing Variables in Large Area Polymer Sintering of Single Layer Components. In Proceedings of the Solid Freeform Fabrication, Austin, TX, USA, 8–10 August 2016. [Google Scholar]
- Ziaee, M.; Tridas, E.M.; Crane, N.B. Binder-Jet Printing of Fine Stainless Steel Powder with Varied Final Density. JOM 2017, 69, 592–596. [Google Scholar] [CrossRef]
- Jacob, G.; Donmez, A.; Slotwinski, J.; Moylan, S. Measurement of powder bed density in powder bed fusion additive manufacturing processes. Meas. Sci. Technol. 2016, 27, 115601. [Google Scholar] [CrossRef]
- Schure, M.R.; Maier, R.S. How does column packing microstructure affect column efficiency in liquid chromatography? J. Chromatogr. A 2006, 1126, 58–69. [Google Scholar] [CrossRef]
- Daneyko, A.; Höltzel, A.; Khirevich, S.; Tallarek, U. Influence of the particle size distribution on hydraulic permeability and eddy dispersion in bulk packings. Anal. Chem. 2011, 83, 3903–3910. [Google Scholar] [CrossRef]
- Daneyko, A.; Khirevich, S.; Höltzel, A.; Seidel-Morgenstern, A.; Tallarek, U. From random sphere packings to regular pillar arrays: Effect of the macroscopic confinement on hydrodynamic dispersion. J. Chromatogr. A 2011, 1218, 8231–8248. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Haeri, S. Optimisation of blade type spreaders for powder bed preparation in Additive Manufacturing using DEM simulations. Powder Technol. 2017, 321, 94–104. [Google Scholar] [CrossRef]
- Sungail, C.; Abid, A. Spherical tantalum feed powder for metal additive manufacturing. Met. Powder Rep. 2018, 73, 316–318. [Google Scholar] [CrossRef]
- Avinash, B.S.; Chaturmukha, V.S.; Jayanna, H.S.; Naveen, C.S.; Rajeeva, M.P.; Harish, B.M.; Suresh, S.; Lamani, A.R. Effect of particle size on band gap and DC electrical conductivity of TiO2 nanomaterial. AIP Conf. Proc. 2016, 1728, 020426. [Google Scholar]
- Bembey, A.; Bushby, A.; Boyde, A.; Ferguson, V.; Oyen, M. Hydration effects on the micro-mechanical properties of bone. J. Mater. Res. 2006, 21, 1962–1968. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaee, M.; Hershman, R.; Mahmood, A.; Crane, N.B. Fabrication of Demineralized Bone Matrix/Polycaprolactone Composites Using Large Area Projection Sintering (LAPS). J. Manuf. Mater. Process. 2019, 3, 30. https://doi.org/10.3390/jmmp3020030
Ziaee M, Hershman R, Mahmood A, Crane NB. Fabrication of Demineralized Bone Matrix/Polycaprolactone Composites Using Large Area Projection Sintering (LAPS). Journal of Manufacturing and Materials Processing. 2019; 3(2):30. https://doi.org/10.3390/jmmp3020030
Chicago/Turabian StyleZiaee, Mohsen, Rebecca Hershman, Ayesha Mahmood, and Nathan B. Crane. 2019. "Fabrication of Demineralized Bone Matrix/Polycaprolactone Composites Using Large Area Projection Sintering (LAPS)" Journal of Manufacturing and Materials Processing 3, no. 2: 30. https://doi.org/10.3390/jmmp3020030
APA StyleZiaee, M., Hershman, R., Mahmood, A., & Crane, N. B. (2019). Fabrication of Demineralized Bone Matrix/Polycaprolactone Composites Using Large Area Projection Sintering (LAPS). Journal of Manufacturing and Materials Processing, 3(2), 30. https://doi.org/10.3390/jmmp3020030




