A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part III: Laser Reactive Synthesis within Diamond Anvil Cells
Abstract
:1. Introduction
2. Methodology
3. Overview of Laser Reactive Chemical Synthesis in Diamond Anvil Cells (LRS-DAC) Experimentation
4. LRS-DACs: Physical Processes, Historical Development, and Key Experiments
4.1. Chemical Reactions
4.1.1. Chemical Thermodynamics
4.1.2. Chemical Kinetics:
4.1.3. Diffusive and Convective Mass Transport:
4.2. Diffusive and Convective Heat Transfer
4.3. LRS-DAC: Historical Development & Key Experiments
5. LRS-DAC Synthesis: Summary of Important Material Classes
- Low-Z Carbon-Containing Compounds/Materials.
- Binary Metal Hydrides.
- Binary Metal Borides.
- Binary Metal Carbides.
- Binary Metal Nitrides.
- Binary Metal Oxides.
- High-Z Intermetallics and Metallic Compounds.
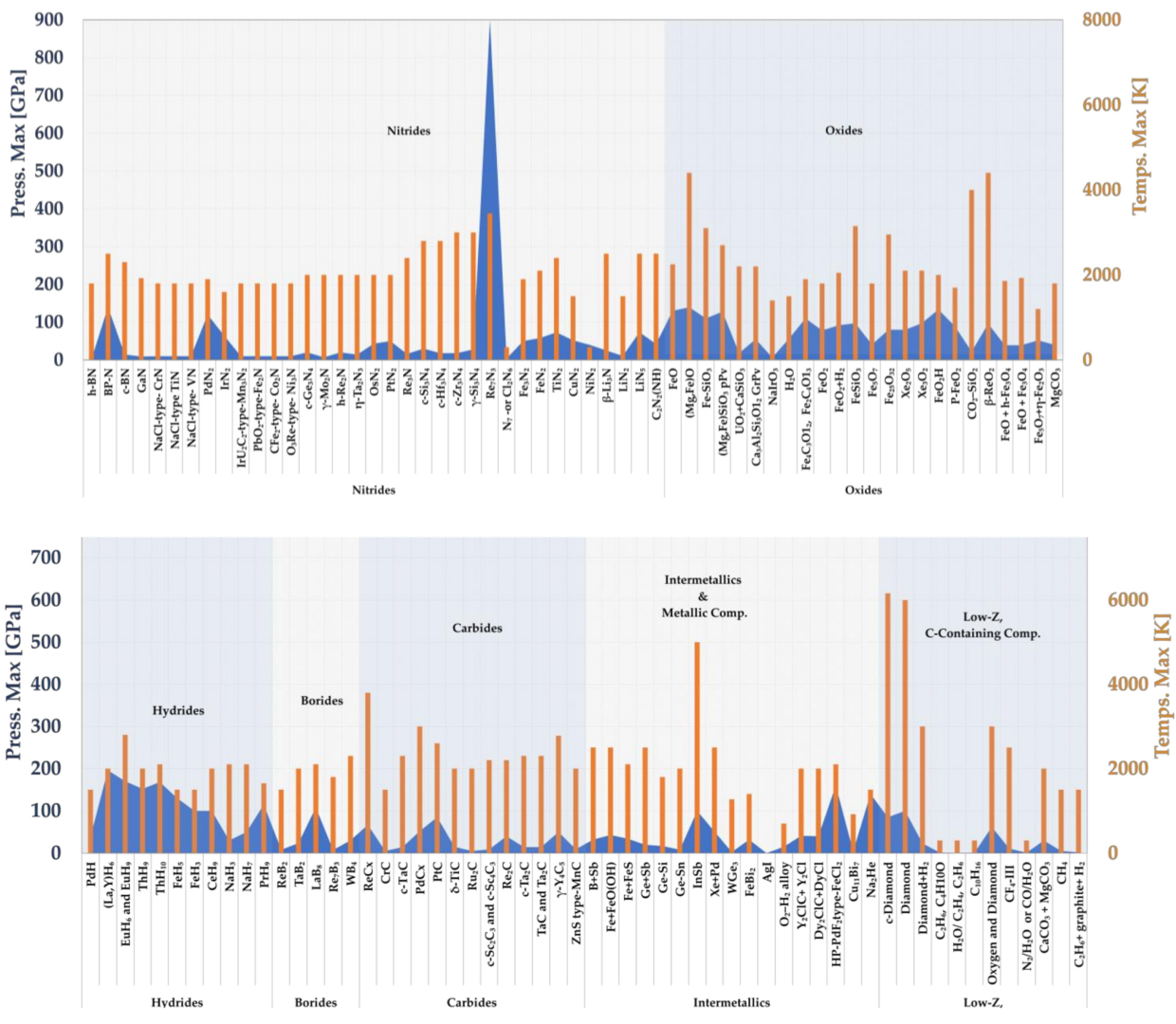
5.1. Low-Z Carbon-Containing Compounds/Materials
5.2. Binary Metal Hydrides
5.3. Binary Metal Borides
5.4. Binary Metal Carbides
- Transition Metal Carbides: The scandium carbides (Sc2C5 and Sc4C3), titanium carbide (TiC), chromium carbide (CrC),
- Refractory Metal Carbides: The titanium carbides (TaC and Ta2C), and rhenium carbides (ReCx and Re2C).
- Platinum Group Metal Carbides: Palladium carbide (PdCx), ruthenium carbide (Ru2C), and platinum carbide (PtC).
5.5. Binary Metal Nitrides
- Metalloid-based Nitrides: Boron nitride (BN), silicon nitride (Si3N4), and germanium nitride (Ge3N4). (See Table 5).
- Transition Metal Nitrides: Manganese nitride (Mn3N2), iron nitride (Fe2N), cobalt nitride (Co2N), nickel nitride (Ni3N), and gallium nitride (GaN). (See Table 6).
- Refractory Metal Nitrides: Titanium nitride (TiN), vanadium nitride (VN), chromium nitride (CrN), zirconium nitride (Zr3N4), molybdenum nitride (Mo2N), hafnium nitride (Hf3N4), tantalum nitride (Ta2N3), and the rhenium nitrides (Re2N and Re3N). (See Table 7).
- Platinum Group Metal Nitrides: Palladium nitride (PdN2), osmium nitride (OsN2), iridium nitride (IrN2), and platinum nitride (PtN2). (See Table 8).
5.6. Binary Metal Oxides
5.7. High-Z Intermetallics and Metallic Compounds
6. Conclusions & Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bini, R.; Schettino, V. Materials Under Extreme Conditions: Molecular Crystals at High Pressure; Imperial College Press: London, UK, 2014; Volume 1, p. 18. [Google Scholar]
- Kestell, A.E.; DeLorey, G.T. Nanoparticles: Properties, Classification, Characterization, and Fabrication: Properties, Classification, Characterization, and Fabrication; Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2010. [Google Scholar]
- Kim, T.; Hyeon, T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology 2013, 25, 012001. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.-H.; Kim, H.-C. A high productivity and speedy synthesis process for copper nanowires via an ethylenediamine-mediated method. Int. Nano Lett. 2017, 7, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Ganachari, S.V.; Banapurmath, N.R.; Salimath, B.; Yaradoddi, J.S.; Shettar, A.S.; Hunashyal, A.M.; Venkataraman, A.; Patil, P.; Shoba, H.; Hiremath, G.B. Synthesis Techniques for Preparation of Nanomaterials. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 83–103. [Google Scholar]
- Ma, L.; Zhou, M.; Wang, Y.; Kawaguchi, S.; Ohishi, Y.; Peng, F.; Liu, H.; Liu, G.; Wang, H.; Ma, Y. Experimental clathrate superhydrides EuH6 and EuH9 at extreme pressure conditions. Phys. Rev. Res. 2021, 3, 043107. [Google Scholar] [CrossRef]
- Arpita Aparajita, A.N.; Sanjay Kumar, N.R.; Chandra, S.; Amirthapandian, S.; Shekar, N.V.C.; Sridhar, K. High-Pressure Synthesis of Manganese Monocarbide: A Potential Superhard Material. Inorg. Chem. 2018, 57, 14178–14185. [Google Scholar] [CrossRef] [PubMed]
- Bayarjargal, L.; Morgenroth, W.; Schrodt, N.; Winkler, B.; Milman, V.; Stanek, C.R.; Uberuaga, B.P. Synthesis of Hf8O7, a new binary hafnium oxide, at high pressures and high temperatures. High Press. Res. 2017, 37, 147–158. [Google Scholar] [CrossRef]
- Watkins, E.B.; Huber, R.C.; Childs, C.M.; Salamat, A.; Pigott, J.S.; Chow, P.; Xiao, Y.; Coe, J.D. Diamond and methane formation from the chemical decomposition of polyethylene at high pressures and temperatures. Sci. Rep. 2022, 12, 631. [Google Scholar] [CrossRef]
- Robertson, J. 3.06—Diamond-Like Carbon Films, Properties and Applications. In Comprehensive Hard Materials; Sarin, V.K., Ed.; Elsevier: Oxford, UK, 2014; pp. 101–139. [Google Scholar]
- Taniguchi, T. 3.23—Synthesis and Properties of Single Crystalline cBN and Its Sintered Body. In Comprehensive Hard Materials; Sarin, V.K., Ed.; Elsevier: Oxford, UK, 2014; pp. 587–605. [Google Scholar]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- Suzuki, S.; Niwa, K.; Honda, A.; Muto, S.; Ando, A.; Hasegawa, M. High-pressure synthesis and Sn valence state analysis of BaTiO3–SnO solid solution. J. Mater. Res. 2014, 29, 2928–2933. [Google Scholar] [CrossRef]
- Sanjay Kumar, N.R.; Chandra Shekar, N.V.; Chandra, S.; Basu, J.; Divakar, R.; Sahu, P.C. Synthesis of novel Ru2C under high pressure–high temperature conditions. J. Phys. Condens. Matter 2012, 24, 362202. [Google Scholar] [CrossRef]
- Riedel, R.; Horvath-Bordon, E.; Kroll, P.; Miehe, G.; Dzivenko, D.; Kleebe, H.-J.; Aken, P.A.v.; Lauterbach, S. Novel binary and ternary phases in the Si-C-N system. J. Ceram. Soc. Jpn. 2008, 116, 674–680. [Google Scholar] [CrossRef] [Green Version]
- Ratzker, B.; Wagner, A.; Favelukis, B.; Ayalon, I.; Shrem, R.; Kalabukhov, S.; Frage, N. Effect of synthesis route on optical properties of Cr:Al2O3 transparent ceramics sintered under high pressure. J. Alloys Compd. 2022, 913, 165186. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Friedrich, A.; Bayarjargal, L.; Milman, V.; Yan, J.; Clark, S.M. Stability field of the high-(P, T) Re2C phase and properties of an analogous osmium carbide phase. J. Alloys Compd. 2009, 481, 577–581. [Google Scholar] [CrossRef]
- Ma, L.; Yang, X.; Liu, G.; Liu, H.; Yang, G.; Wang, H.; Cai, J.; Zhou, M.; Wang, H. Design and synthesis of clathrate LaB8 with superconductivity. Phys. Rev. B 2021, 104, 174112. [Google Scholar] [CrossRef]
- Arpita Aparajita, A.N.; Sanjay Kumar, N.R.; Chandra, S.; Chandra Shekar, N.V.; Kalavathi, S. Synthesis of novel chromium carbide using laser heated diamond anvil cell. J. Solid State Chem. 2021, 295, 121899. [Google Scholar] [CrossRef]
- Aslandukova, A.; Aslandukov, A.; Yuan, L.; Laniel, D.; Khandarkhaeva, S.; Fedotenko, T.; Steinle-Neumann, G.; Glazyrin, K.; Dubrovinskaia, N.; Dubrovinsky, L. Novel High-Pressure Yttrium Carbide γ-Y4C5 Containing [C2] and Nonlinear [C3] Units with Unusually Large Formal Charges. Phys. Rev. Lett. 2021, 127, 135501. [Google Scholar] [CrossRef]
- Daviau, K.; Lee, K.K.M. Experimental Constraints on Solid Nitride Phases in Rocky Mantles of Reduced Planets and Implications for Observable Atmosphere Compositions. J. Geophys. Res. Planets 2021, 126, e2020JE006687. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H.; Niu, C.; Wang, X.; Zhang, J.; Ge, Z.; Li, Y. Synthesis of black phosphorus structured polymeric nitrogen. Chin. Phys. B 2020, 29, 106201. [Google Scholar] [CrossRef]
- Kronbo, C.H.; Ottesen, M.; Hansen, M.F.; Ehrenreich-Petersen, E.; Meng, Y.; Bremholm, M. Discovery of Rhombohedral NaIrO3 Polymorph by In Situ High-Pressure Synthesis of High-Oxidation-State Materials Using Laser Heating in Diamond Anvil Cells. Inorg. Chem. 2020, 59, 15780–15787. [Google Scholar] [CrossRef]
- Walsh, J.P.S.; Freedman, D.E. High-Pressure Synthesis: A New Frontier in the Search for Next-Generation Intermetallic Compounds. Acc. Chem. Res. 2018, 51, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Tamerius, A.D.; Clarke, S.M.; Gu, M.; Walsh, J.P.S.; Esters, M.; Meng, Y.; Hendon, C.H.; Rondinelli, J.M.; Jacobsen, S.D.; Freedman, D.E. Discovery of Cu3Pb. Angew. Chem. Int. Ed. 2018, 57, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells. J. Manuf. Mater. Process. 2022, 6, 111. [Google Scholar] [CrossRef]
- Bassett, W.A. Diamond anvil cell, 50th birthday. High Press. Res. 2009, 29, 163–186. [Google Scholar] [CrossRef]
- Shukla, B.; Shekar, N.V.C.; Kumar, N.R.S.; Ravindran, T.R.; Sahoo, P.; Dhara, S.; Sahu, P.C. Twin chamber sample assembly in DAC and HPHT studies on GaN nano-particles. J. Phys. Conf. Ser. 2012, 377, 012014. [Google Scholar] [CrossRef]
- Shim, S.H.; Duffy, T.S.; Jeanloz, R.; Shen, G. Stability and crystal structure of MgSiO3 perovskite to the core-mantle boundary. Geophys. Res. Lett. 2004, 31, GL019639. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; McGuire, C.; Makhluf, A.; Kavner, A.; Chuliá-Jordán, R.; Pellicer-Porres, J.; Martinez-García, D.; Doran, A.; Kunz, M.; Rodríguez-Hernández, P.; et al. Exploring the Chemical Reactivity between Carbon Dioxide and Three Transition Metals (Au, Pt, and Re) at High-Pressure, High-Temperature Conditions. Inorg. Chem. 2016, 55, 10793–10799. [Google Scholar] [CrossRef] [Green Version]
- Chuliá-Jordán, R.; Santamaría-Pérez, D.; Marqueño, T.; Ruiz-Fuertes, J.; Daisenberger, D. Oxidation of High Yield Strength Metals Tungsten and Rhenium in High-Pressure High-Temperature Experiments of Carbon Dioxide and Carbonates. Crystals 2019, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Beyer, M.K.; Clausen-Schaumann, H. Mechanochemistry: The Mechanical Activation of Covalent Bonds. Chem. Rev. 2005, 105, 2921–2948. [Google Scholar] [CrossRef]
- Cruz, C. Ultrafast Spectroscopy of Force- or Light-induced Chemical and Electronic Changes in Condensed Phase Materials. Ph.D. Thesis, University of California, Ann Arbor, MI, USA, 2019. [Google Scholar]
- Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part II: Laser-Driven Dynamic Compression within Diamond Anvil Cells. J. Manuf. Mater. Process. 2022, 6, 142. [Google Scholar] [CrossRef]
- Dadashev, A.; Pasternak, M.P.; Rozenberg, G.K.; Taylor, R.D. Applications of perforated diamond anvils for very high-pressure research. Rev. Sci. Instrum. 2001, 72, 2633–2637. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.K. High-pressure studies with x-rays using diamond anvil cells. Rep. Prog. Phys. 2016, 80, 016101. [Google Scholar] [CrossRef] [Green Version]
- Lenz, J.A.; Perottoni, C.A.; Balzaretti, N.M.; Jornada, J.A.H.d. Processing of amorphous carbon films by ultrafast temperature treatment in a confined geometry. J. Appl. Phys. 2001, 89, 8284–8290. [Google Scholar] [CrossRef] [Green Version]
- Jingzhu, H.; Jian, X.; Somayazulu, M.; Guo, Q.; Hemley, R.; Mao, H.K. X-ray diffraction and laser heating: Application of a moissanite anvil cell. J. Phys. Condens. Matter 2002, 14, 10479. [Google Scholar] [CrossRef]
- Xu, J.-a.; Mao, H.-k.; Hemley, R.J. The gem anvil cell: High-pressure behaviour of diamond and related materials. J. Phys. Condens. Matter 2002, 14, 11549–11552. [Google Scholar] [CrossRef]
- Kimura, T.; Ozaki, N.; Okuchi, T.; Terai, T.; Sano, T.; Shimizu, K.; Sano, T.; Koenig, M.; Hirose, A.; Kakeshita, T.; et al. Significant static pressure increase in a precompression cell target for laser-driven advanced dynamic compression experiments. Phys. Plasma 2010, 17, 054502. [Google Scholar] [CrossRef]
- Patel, N.N.; Sunder, M.; Sharma, S.M. Laser heated diamond anvil cell facility for high temperature high pressure research: Application to material synthesis and melting studies. Indian J. Phys. 2018, 92, 1259–1269. [Google Scholar] [CrossRef]
- Badding, J.V.; Nesting, D.C. Thermodynamic Analysis of the Formation of Carbon Nitrides under Pressure. Chem. Mater. 1996, 8, 535–540. [Google Scholar] [CrossRef]
- Zhang, R. Pressure Effects on Crystal and Electronic Structures of Materials. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 2018. [Google Scholar]
- Drickamer, H.G.; Frank, C.W. Shifts of Energy Levels with Pressure. In Electronic Transitions and the High Pressure Chemistry and Physics of Solids; Springer: Dordrecht, The Netherlands, 1973; pp. 72–109. [Google Scholar]
- Miao, M.; Sun, Y.; Zurek, E.; Lin, H. Chemistry under high pressure. Nat. Rev. Chem. 2020, 4, 508–527. [Google Scholar] [CrossRef]
- Jeanloz, R.; Celliers Peter, M.; Collins Gilbert, W.; Eggert Jon, H.; Lee Kanani, K.M.; McWilliams, R.S.; Brygoo, S.; Loubeyre, P. Achieving high-density states through shock-wave loading of precompressed samples. Proc. Natl. Acad. Sci. USA 2007, 104, 9172–9177. [Google Scholar] [CrossRef] [Green Version]
- Horvath-Bordon, E.; Riedel, R.; Zerr, A.; McMillan, P.F.; Auffermann, G.; Prots, Y.; Bronger, W.; Kniep, R.; Kroll, P. High-pressure chemistry of nitride-based materials. Chem. Soc. Rev. 2006, 35, 987–1014. [Google Scholar] [CrossRef]
- Benedetti, L.R.; Nguyen, J.H.; Caldwell, W.A.; Liu, H.; Kruger, M.; Jeanloz, R. Dissociation of CH4 at High Pressures and Temperatures: Diamond Formation in Giant Planet Interiors? Science 1999, 286, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Zerr, A.; Miehe, G.; Riedel, R. Synthesis of cubic zirconium and hafnium nitride having Th3P4 structure. Nat. Mater. 2003, 2, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Somayazulu, M.; Ahart, M.; Mishra, A.K.; Geballe, Z.M.; Baldini, M.; Meng, Y.; Struzhkin, V.V.; Hemley, R.J. Evidence for Superconductivity above 260 K in Lanthanum Superhydride at Megabar Pressures. Phys. Rev. Lett. 2019, 122, 027001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdov, A.P.; Kong, P.P.; Minkov, V.S.; Besedin, S.P.; Kuzovnikov, M.A.; Mozaffari, S.; Balicas, L.; Balakirev, F.F.; Graf, D.E.; Prakapenka, V.B.; et al. Superconductivity at 250 K in lanthanum hydride under high pressures. Nature 2019, 569, 528–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowhurst, J.C.; Goncharov, A.F.; Sadigh, B.; Evans, C.L.; Morrall, P.G.; Ferreira, J.L.; Nelson, A.J. Synthesis and Characterization of the Nitrides of Platinum and Iridium. Science 2006, 311, 1275–1278. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.-k. Synthesis of Novel Transition Metal Nitrides IrN2 and OsN. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef] [Green Version]
- Gregoryanz, E.; Sanloup, C.; Somayazulu, M.; Badro, J.; Fiquet, G.; Mao, H.-K.; Hemley, R.J. Synthesis and characterization of a binary noble metal nitride. Nat. Mater. 2004, 3, 294–297. [Google Scholar] [CrossRef]
- Hu, Q.; Kim, D.Y.; Yang, W.; Yang, L.; Meng, Y.; Zhang, L.; Mao, H.K. FeO2 and FeOOH under deep lower-mantle conditions and Earth’s oxygen-hydrogen cycles. Nature 2016, 534, 241–244. [Google Scholar] [CrossRef]
- Goncharov, A.F.; Goldman, N.; Fried, L.E.; Crowhurst, J.C.; Kuo, I.F.W.; Mundy, C.J.; Zaug, J.M. Dynamic Ionization of Water under Extreme Conditions. Phys. Rev. Lett. 2005, 94, 125508. [Google Scholar] [CrossRef] [Green Version]
- Ono, S.; Kikegawa, T.; Ohishi, Y. A high-pressure and high-temperature synthesis of platinum carbide. Solid State Commun. 2005, 133, 55–59. [Google Scholar] [CrossRef]
- Biellmann, C.; Gillet, P.; Guyot, F.o.; Peyronneau, J.; Reynard, B. Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet. Sci. Lett. 1993, 118, 31–41. [Google Scholar] [CrossRef]
- Crowhurst, J.C.; Goncharov, A.F.; Sadigh, B.; Zaug, J.M.; Aberg, D.; Meng, Y.; Prakapenka, V.B. Synthesis and characterization of nitrides of iridium and palladium. J. Mater. Res. 2008, 23, 1–5. [Google Scholar] [CrossRef]
- Semenok, D.V.; Kvashnin, A.G.; Ivanova, A.G.; Svitlyk, V.; Fominski, V.Y.; Sadakov, A.V.; Sobolevskiy, O.A.; Pudalov, V.M.; Troyan, I.A.; Oganov, A.R. Superconductivity at 161 K in thorium hydride ThH10: Synthesis and properties. Mater. Today 2020, 33, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Serghiou, G.; Miehe, G.; Tschauner, O.; Zerr, A.; Boehler, R. Synthesis of a cubic Ge3N4 phase at high pressures and temperatures. J. Chem. Phys. 1999, 111, 4659–4662. [Google Scholar] [CrossRef]
- Friedrich, A.; Winkler, B.; Bayarjargal, L.; Morgenroth, W.; Juarez-Arellano, E.A.; Milman, V.; Refson, K.; Kunz, M.; Chen, K. Novel Rhenium Nitrides. Phys. Rev. Lett. 2010, 105, 085504. [Google Scholar] [CrossRef] [PubMed]
- Citroni, M.; Ceppatelli, M.; Bini, R.; Schettino, V. Laser-Induced Selectivity for Dimerization Versus Polymerization of Butadiene Under Pressure. Science 2002, 295, 2058–2060. [Google Scholar] [CrossRef] [PubMed]
- Bykova, E.; Dubrovinsky, L.; Dubrovinskaia, N.; Bykov, M.; McCammon, C.; Ovsyannikov, S.V.; Liermann, H.P.; Kupenko, I.; Chumakov, A.I.; Rüffer, R.; et al. Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 2016, 7, 10661. [Google Scholar] [CrossRef] [Green Version]
- Bykov, M.; Bykova, E.; Aprilis, G.; Glazyrin, K.; Koemets, E.; Chuvashova, I.; Kupenko, I.; McCammon, C.; Mezouar, M.; Prakapenka, V.; et al. Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains. Nat. Commun. 2018, 9, 2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takafuji, N.; Hirose, K.; Mitome, M.; Bando, Y. Solubilities of O and Si in liquid iron in equilibrium with (Mg,Fe)SiO3 perovskite and the light elements in the core. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Pépin, C.M.; Geneste, G.; Dewaele, A.; Mezouar, M.; Loubeyre, P. Synthesis of FeH5: A layered structure with atomic hydrogen slabs. Science 2017, 357, 382–385. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, W.A.; Nguyen, J.H.; Pfrommer, B.G.; Mauri, F.; Louie, S.G.; Jeanloz, R. Structure, Bonding, and Geochemistry of Xenon at High Pressures. Science 1997, 277, 930–933. [Google Scholar] [CrossRef]
- Friedrich, A.; Winkler, B.; Juarez-Arellano, E.A.; Bayarjargal, L. Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations. Materials 2011, 4, 1648–1692. [Google Scholar] [CrossRef] [Green Version]
- Kolesnikov, A.; Kutcherov, V.G.; Goncharov, A.F. Methane-derived hydrocarbons produced under upper-mantle conditions. Nat. Geosci. 2009, 2, 566–570. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, T. Systematic Study of Formation and Crystal Structure of 3d-Transition Metal Nitrides Synthesized in a Supercritical Nitrogen Fluid under 10 GPa and 1800 K Using Diamond Anvil Cell and YAG Laser Heating. ChemInform 2006, 37, 131–142. [Google Scholar] [CrossRef]
- Dong, X.; Oganov, A.R.; Goncharov, A.F.; Stavrou, E.; Lobanov, S.; Saleh, G.; Qian, G.-R.; Zhu, Q.; Gatti, C.; Deringer, V.L.; et al. A stable compound of helium and sodium at high pressure. Nat. Chem. 2017, 9, 440–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, B.; Duffy, T.S. Finite element simulations of the laser-heated diamond-anvil cell. J. Appl. Phys. 2005, 97, 114902. [Google Scholar] [CrossRef] [Green Version]
- Citroni, M.; Ceppatelli, M.; Bini, R.; Schettino, V. Dimerization and Polymerization of Isoprene at High Pressures. J. Phys. Chem. B 2007, 111, 3910–3917. [Google Scholar] [CrossRef]
- Hirai, H.; Konagai, K.; Kawamura, T.; Yamamoto, Y.; Yagi, T. Polymerization and diamond formation from melting methane and their implications in ice layer of giant planets. Phys. Earth Planet. Inter. 2009, 174, 242–246. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Bini, R.; Schettino, V. High-Pressure Reactivity of Model Hydrocarbons Driven by Near-UV Photodissociation of Water. J. Phys. Chem. B 2009, 113, 14640–14647. [Google Scholar] [CrossRef]
- Peiris, S.M.; Russell, T.P. Photolysis of Compressed Sodium Azide (NaN3) as a Synthetic Pathway to Nitrogen Materials. J. Phys. Chem. A 2003, 107, 944–947. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Fanetti, S.; Citroni, M.; Bini, R. Photoinduced Reactivity of Liquid Ethanol at High Pressure. J. Phys. Chem. B 2010, 114, 15437–15444. [Google Scholar] [CrossRef]
- Zerr, A.; Serghiou, G.; Boehler, R.; Ross, M. Decomposition of alkanes at high pressures and temperatures. High Press. Res. 2006, 26, 23–32. [Google Scholar] [CrossRef]
- Tschauner, O.; Mao, H.-k.; Hemley, R.J. New Transformations of CO2 at High Pressures and Temperatures. Phys. Rev. Lett. 2001, 87, 075701. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, S.M.; Nabiei, F.; Boukaré, C.-E.; Prakapenka, V.B.; Cantoni, M.; Badro, J.; Gillet, P. Composition and Pressure Effects on Partitioning of Ferrous Iron in Iron-Rich Lower Mantle Heterogeneities. Minerals 2021, 11, 512. [Google Scholar] [CrossRef]
- Aprilis, G.; Pakhomova, A.; Chariton, S.; Khandarkhaeva, S.; Melai, C.; Bykova, E.; Bykov, M.; Fedotenko, T.; Koemets, E.; McCammon, C.; et al. The Effect of Pulsed Laser Heating on the Stability of Ferropericlase at High Pressures. Minerals 2020, 10, 542. [Google Scholar] [CrossRef]
- Bayarjargal, L.; Shumilova, T.G.; Friedrich, A.; Winkler, B.R. Diamond formation from CaCO3 at high pressure and temperature. Eur. J. Mineral. 2010, 22, 29–34. [Google Scholar] [CrossRef]
- Dubrovinsky, L.; Khandarkhaeva, S.; Fedotenko, T.; Laniel, D.; Bykov, M.; Giacobbe, C.; Lawrence Bright, E.; Sedmak, P.; Chariton, S.; Prakapenka, V.; et al. Materials synthesis at terapascal static pressures. Nature 2022, 605, 274–278. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Park, S.; Choi, Y.K. Numerical Analysis on Heat Transfer Characteristics of A Silicon Film Irradiated by Pico-To Femtosecond Pulse Lasers. Numer. Heat Transf. Part A 2003, 44, 833–850. [Google Scholar] [CrossRef]
- Karu, T.I.; Tiphlova, O.A.; Matveyets Yu, A.; Yartsev, A.P.; Letokhov, V.S. Comparison of the effects of visible femtosecond laser pulses and continuous wave laser radiation of low average intensity on the clonogenicity of Escherichia coli. J. Photochem. Photobiol. B 1991, 10, 339–344. [Google Scholar] [CrossRef]
- Du, Z.; Amulele, G.; Benedetti, L.R.; Lee, K.K. Mapping temperatures and temperature gradients during flash heating in a diamond-anvil cell. Rev. Sci. Instrum. 2013, 84, 075111. [Google Scholar] [CrossRef]
- Bunn, H.A.; Schultz, C.P.; Jernigan, C.M.; Widicus Weaver, S.L. Laser-Induced Chemistry Observed during 248 nm Vacuum Ultraviolet Photolysis of an O3 and CH3NH2 Mixture. J. Phys. Chem. A 2020, 124, 10838–10848. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, L.; Chen, Y.; Yu, N.; Wang, L.; Huang, K.; Zhao, Y.; Han, G. Long wavelength single photon like driven photolysis via triplet triplet annihilation. Nat. Commun. 2021, 12, 122. [Google Scholar] [CrossRef] [PubMed]
- Toyota, K.; Nakashima, S.; Okada, T. Near-infrared laser-induced breakdown of liquid benzene. Chem. Phys. Lett. 2000, 323, 323–328. [Google Scholar] [CrossRef]
- Wallace, M. High-Pressure High-Temperature (HPHT) Synthesis of Functional Materials. In Sintering of Functional Materials; Igor, S., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 8. [Google Scholar]
- Tokita, M. Progress of Spark Plasma Sintering (SPS) Method, Systems, Ceramics Applications and Industrialization. Ceramics 2021, 4, 160–198. [Google Scholar] [CrossRef]
- Stokes, C.S. Chemistry in High Temperature Plasma Jets. In Chemical Reactions in Electrical Discharges; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1969; Volume 80, pp. 390–405. [Google Scholar]
- Ridha, F.; Junus, S.; Darsin, M. Synthesis of Zinc Oxide (ZnO) Nanoparticle using Non-Transferred DC Thermal Plasma Method: A Morphology Review. Int. J. Emerg. Trends Eng. Res. 2021, 9, 983–987. [Google Scholar] [CrossRef]
- Elaissi, S.; Ben Gouider Trabelsi, A.; Alkallas, F.H.; Alrebdi, T.A.; Charrada, K. Modeling of Advanced Silicon Nanomaterial Synthesis Approach: From Reactive Thermal Plasma Jet to Nanosized Particles. Nanomaterials 2022, 12, 1763. [Google Scholar] [CrossRef]
- Yoo, C.S.; Akella, J.; Cynn, H.; Nicol, M. Direct elementary reactions of boron and nitrogen at high pressures and temperatures. Phys. Rev. B Condens. Matter 1997, 56, 140–146. [Google Scholar] [CrossRef]
- Sahu, P.C.; Takemura, K.; Yusa, H. Synthesis experiments on B-Sb, Ge-Sb, and Xe-Pd systems using a laser heated diamond anvil cell. High Press. Res. 2001, 21, 41–50. [Google Scholar] [CrossRef]
- Sorb, Y.A.; Subramanian, N.; Ravindran, T.R.; Sahu, P.C. High Pressure in situ Micro-Raman Spectroscopy of Ge-Sn System Synthesized in a Laser Heated Diamond Anvil Cell. AIP Conf. Proc. 2011, 1349, 1305–1306. [Google Scholar] [CrossRef]
- Fedotenko, T.; Dubrovinsky, L.; Khandarkhaeva, S.; Chariton, S.; Koemets, E.; Koemets, I.; Hanfland, M.; Dubrovinskaia, N. Synthesis of palladium carbides and palladium hydride in laser heated diamond anvil cells. J. Alloys Compd. 2020, 844, 156179. [Google Scholar] [CrossRef]
- Gréaux, S.; Andrault, D.; Gautron, L.; Bolfan-Casanova, N.; Mezouar, M. Compressibility of Ca3Al2Si3O12 perovskite up to 55 GPa. Phys. Chem. Miner. 2014, 41, 419–429. [Google Scholar] [CrossRef]
- Semenok, D.V.; Troyan, I.A.; Ivanova, A.G.; Kvashnin, A.G.; Kruglov, I.A.; Hanfland, M.; Sadakov, A.V.; Sobolevskiy, O.A.; Pervakov, K.S.; Lyubutin, I.S.; et al. Superconductivity at 253 K in lanthanum–yttrium ternary hydrides. Mater. Today 2021, 48, 18–28. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Bini, R.; Schettino, V. High-pressure reactivity of clathrate hydrates by two-photon dissociation of water. Phys. Chem. Chem. Phys. 2011, 13, 1264–1275. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Bini, R.; Schettino, V. High-pressure photodissociation of water as a tool for hydrogen synthesis and fundamental chemistry. Proc. Natl. Acad. Sci. USA 2009, 106, 11454–11459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, W.L.; Mao, H.-k.; Meng, Y.; Eng, P.J.; Hu, M.Y.; Chow, P.; Cai, Y.Q.; Shu, J.; Hemley, R.J. X-ray–Induced Dissociation of H2O and Formation of an O2–H2 Alloy at High Pressure. Science 2006, 314, 636–638. [Google Scholar] [CrossRef]
- Duffy, T.S.; Smith, R.F. Ultra-High Pressure Dynamic Compression of Geological Materials. Front. Earth Sci. 2019, 7. [Google Scholar] [CrossRef]
- Yoo, C.-S.; Wei, H.; Dias, R.; Shen, G.; Smith, J.; Chen, J.-Y.; Evans, W. Time-Resolved Synchrotron X-ray Diffraction on Pulse Laser Heated Iron in Diamond Anvil Cell. J. Phys. Conf. Ser. 2012, 377, 012108. [Google Scholar] [CrossRef] [Green Version]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. High melting points of tantalum in a laser-heated diamond anvil cell. Phys. Rev. Lett. 2010, 104, 255701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrault, D.; Fiquet, G.; Kunz, M.; Visocekas, F.; Häusermann, D. The Orthorhombic Structure of Iron: An in Situ Study at High-Temperature and High-Pressure. Science 1997, 278, 831–834. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.S.; Walter, M.J. Tetragonal almandine pyrope phase (TAPP): Retrograde Mg-perovskite from subducted oceanic crust? Eur. J. Mineral. 2012, 24, 587–597. [Google Scholar] [CrossRef]
- Armstrong, L.S.; Walter, M.J.; Tuff, J.R.; Lord, O.T.; Lennie, A.R.; Kleppe, A.K.; Clark, S.M. Perovskite Phase Relations in the System CaO–MgO–TiO2–SiO2 and Implications for Deep Mantle Lithologies. J. Petrol. 2012, 53, 611–635. [Google Scholar] [CrossRef] [Green Version]
- Benedetti, L.R.; Loubeyre, P. Temperature gradients, wavelength-dependent emissivity, and accuracy of high and very-high temperatures measured in the laser-heated diamond cell. High Press. Res. 2004, 24, 423–445. [Google Scholar] [CrossRef]
- Boehler, R. Melting of the FeFeO and the FeFeS systems at high pressure: Constraints on core temperatures. Earth Planet. Sci. Lett. 1992, 111, 217–227. [Google Scholar] [CrossRef]
- Boehler, R. Temperatures in the Earth’s core from melting-point measurements of iron at high static pressures. Nature 1993, 363, 534–536. [Google Scholar] [CrossRef]
- Boehler, R.; Ross, M.; Boercker, D.B. High-pressure melting curves of alkali halides. Phys. Rev. B Condens. Matter 1996, 53, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Boehler, R.; Chopelas, A. A new approach to laser heating in high pressure mineral physics. Geophys. Res. Lett. 1991, 18, 1147–1150. [Google Scholar] [CrossRef]
- Boehler, R.; Ross, M.; Boercker, D.B. Melting of LiF and NaCl to 1 Mbar: Systematics of Ionic Solids at Extreme Conditions. Phys. Rev. Lett. 1997, 78, 4589–4592. [Google Scholar] [CrossRef]
- Bolfan-Casanova, N.; Andrault, D.; Amiguet, E.; Guignot, N. Equation of state and post-stishovite transformation of Al-bearing silica up to 100GPa and 3000K. Phys. Earth Planet. Inter. 2009, 174, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Meade, C.; Mao, H.K.; Hu, J. High-Temperature Phase Transition and Dissociation of (Mg, Fe)SiO3 Perovskite at Lower Mantle Pressures. Science 1995, 268, 1743–1745. [Google Scholar] [CrossRef]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. Melting of lead under high pressure studied using second-scale time-resolved x-ray diffraction. Phys. Rev. B Condens. Matter 2007, 76, 144106. [Google Scholar] [CrossRef]
- Dewaele, A.; Belonoshko, A.B.; Garbarino, G.; Occelli, F.; Bouvier, P.; Hanfland, M.; Mezouar, M. High-pressure--high-temperature equation of state of KCl and KBr. Phys. Rev. B Condens. Matter 2012, 85, 214105. [Google Scholar] [CrossRef]
- Dobson, D.P.; Hunt, S.A.; Ahmed, J.; Lord, O.T.; Wann, E.T.H.; Santangeli, J.; Wood, I.G.; Vočadlo, L.; Walker, A.M.; Thomson, A.R.; et al. The phase diagram of NiSi under the conditions of small planetary interiors. Phys. Earth Planet. Inter. 2016, 261, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Dubrovinsky, L.S.; Saxena, S.K.; Lazor, P.; Ahuja, R.; Eriksson, O.; Wills, J.M.; Johansson, B. Experimental and theoretical identification of a new high-pressure phase of silica. Nature 1997, 388, 362–365. [Google Scholar] [CrossRef]
- Errandonea, D.; Schwager, B.; Ditz, R.; Gessmann, C.; Boehler, R.; Ross, M. Systematics of transition-metal melting. Phys. Rev. B Condens. Matter 2001, 63, 132104. [Google Scholar] [CrossRef]
- Errandonea, D.; Somayazulu, M.; Häusermann, D.; Mao, H.K. Melting of tantalum at high pressure determined by angle dispersive x-ray diffraction in a double-sided laser-heated diamond-anvil cell. J. Phys. Condens. Matter 2003, 15, 7635–7649. [Google Scholar] [CrossRef]
- Errandonea, D.; Boehler, R.; Japel, S.; Mezouar, M.; Benedetti, L.R. Structural transformation of compressed solid Ar: An X-ray diffraction study to 114 GPa. Phys. Rev. B Condens. Matter 2006, 73, 092106. [Google Scholar] [CrossRef]
- Errandonea, D.; MacLeod, S.G.; Burakovsky, L.; Santamaria-Perez, D.; Proctor, J.E.; Cynn, H.; Mezouar, M. Melting curve and phase diagram of vanadium under high-pressure and high-temperature conditions. Phys. Rev. B Condens. Matter 2019, 100, 094111. [Google Scholar] [CrossRef] [Green Version]
- Fedotenko, T.; Dubrovinsky, L.; Aprilis, G.; Koemets, E.; Snigirev, A.; Snigireva, I.; Barannikov, A.; Ershov, P.; Cova, F.; Hanfland, M.; et al. Laser heating setup for diamond anvil cells for in situ synchrotron and in house high and ultra-high pressure studies. Rev. Sci. Instrum. 2019, 90, 104501–104511. [Google Scholar] [CrossRef]
- Fiquet, G.; Andrault, D. Powder X-ray diffraction under extreme conditions of pressure and temperature. J. Synchrotron Radiat. 1999, 6, 81–86. [Google Scholar] [CrossRef]
- Fiquet, G.; Andrault, D.; Itié, J.P.; Gillet, P.; Richet, P. X-ray diffraction of periclase in a laser-heated diamond-anvil cell. Phys. Earth Planet. Inter. 1996, 95, 1–17. [Google Scholar] [CrossRef]
- Fiquet, G.; Dewaele, A.; Andrault, D.; Kunz, M.; Le Bihan, T. Thermoelastic properties and crystal structure of MgSiO3 perovskite at lower mantle pressure and temperature conditions. Geophys. Res. Lett. 2000, 27, 21–24. [Google Scholar] [CrossRef]
- Friedrich, A.; Morgenroth, W.; Bayarjargal, L.; Juarez-Arellano, E.A.; Winkler, B.; Konôpková, Z. In situ study of the high pressure high-temperature stability field of TaN and of the compressibilities of ϑ-TaN and TaON. High Press. Res. 2013, 33, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Golberg, D.; Bando, Y.; Eremets, M.; Takemura, K.; Kurashima, K.; Yusa, H. Nanotubes in boron nitride laser heated at high pressure. Appl. Phys. Lett. 1996, 69, 2045–2047. [Google Scholar] [CrossRef]
- Heinz, D.L.; Sweeney, J.S.; Miller, P. A laser heating system that stabilizes and controls the temperature: Diamond anvil cell applications. Rev. Sci. Instrum. 1991, 62, 1568–1575. [Google Scholar] [CrossRef]
- Hrubiak, R.; Meng, Y.; Shen, G. Microstructures define melting of molybdenum at high pressures. Nat. Commun. 2017, 8, 14562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Li, F.; Zhou, Q.; Meng, Y.; Litasov, K.D.; Wang, X.; Liu, B.; Cui, T. Thermal equation of state of Molybdenum determined from in situ synchrotron X-ray diffraction with laser-heated diamond anvil cells. Sci. Rep. 2016, 6, 19923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Japel, S.; Schwager, B.; Boehler, R.; Ross, M. Melting of copper and nickel at high pressure: The role of d electrons. Phys. Rev. Lett. 2005, 95, 167801. [Google Scholar] [CrossRef]
- Kavner, A.; Duffy, T.S. Pressure–volume–temperature paths in the laser-heated diamond anvil cell. J. Appl. Phys. 2001, 89, 1907–1914. [Google Scholar] [CrossRef]
- Kesson, S.E.; Fitz Gerald, J.D. Partitioning of MgO, FeO, NiO, MnO and Cr2O3 between magnesian silicate perovskite and magnesiowüstite: Implications for the origin of inclusions in diamond and the composition of the lower mantle. Earth Planet. Sci. Lett. 1992, 111, 229–240. [Google Scholar] [CrossRef]
- Kesson, S.E.; Fitz Gerald, J.D.; Shelley, J.M.G. Mineral chemistry and density of subducted basaltic crust at lower-mantle pressures. Nature 1994, 372, 767–769. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Ming, L.C.; Manghnani, M.H. High-pressure phase transformations in a natural crystalline diopside and a synthetic CaMgSi2O6 glass. Phys. Earth Planet. Inter. 1994, 83, 67–79. [Google Scholar] [CrossRef]
- Kimura, T.; Kuwayama, Y.; Yagi, T. Melting temperatures of H2O up to 72 GPa measured in a diamond anvil cell using CO2 laser heating technique. J. Chem. Phys. 2014, 140, 074501. [Google Scholar] [CrossRef]
- Young-Ho, K.O.; Kyoung Hun, O.H.; Kwang Joo, K.I.M. Installation and Operation of a Double-Sided Laser Heating System for the Synthesis of Novel Materials Under Extreme Conditions. New Phys. Sae Mulli (NPSM) 2019, 69, 1107–1114. [Google Scholar] [CrossRef]
- Knittle, E.; Jeanloz, R. Synthesis and Equation of State of (Mg,Fe) SiO3 Perovskite to Over 100 Gigapascals. Science 1987, 235, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Sanjay, N.R.; Kumar, N.; Chandra Shekar, N.V.; Sekar, M.; Natarajan, S.; Mohan, P.; Srinivasan, M.; Padmanabhan, P.; Sahu, P.C. Diamond and diamond-like carbon in laser heated diamond anvil cell at 16.5 GPa and above 2000 K from pyrolitic graphite. Indian J. Pure Appl. Phys. 2008, 46, 783–787. [Google Scholar]
- Kupenko, I.; Strohm, C.; McCammon, C.; Cerantola, V.; Glazyrin, K.; Petitgirard, S.; Vasiukov, D.; Aprilis, G.; Chumakov, A.I.; Rüffer, R.; et al. Time differentiated nuclear resonance spectroscopy coupled with pulsed laser heating in diamond anvil cells. Rev. Sci. Instrum. 2015, 86, 114501. [Google Scholar] [CrossRef] [Green Version]
- Lavina, B.; Dera, P.; Kim, E.; Meng, Y.; Downs Robert, T.; Weck Philippe, F.; Sutton Stephen, R.; Zhao, Y. Discovery of the recoverable high-pressure iron oxide Fe4O. Proc. Natl. Acad. Sci. USA 2011, 108, 17281–17285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazicki, A.; Dewaele, A.; Loubeyre, P.; Mezouar, M. High-pressure--temperature phase diagram and the equation of state of beryllium. Phys. Rev. B Condens. Matter 2012, 86, 174118. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-F.; Santoro, M.; Struzhkin, V.V.; Mao, H.-k.; Hemley, R.J. In situ high pressure-temperature Raman spectroscopy technique with laser-heated diamond anvil cells. Rev. Sci. Instrum. 2004, 75, 3302–3306. [Google Scholar] [CrossRef]
- Lin, J.-F.; Sturhahn, W.; Zhao, J.; Shen, G.; Mao, H.-K.; Hemley, R.J. Absolute temperature measurement in a laser-heated diamond anvil cell. Geophys. Res. Lett. 2004, 31, L14611. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Hu, Q.; Zhu, L.; Meng, Y. Structure and Stability of Iron Fluoride at High Pressure–Temperature and Implication for a New Reservoir of Fluorine in the Deep Earth. Minerals 2020, 10, 783. [Google Scholar] [CrossRef]
- Liu, L.-G. A new high-pressure phase of spinel. Earth Planet. Sci. Lett. 1978, 41, 398–404. [Google Scholar] [CrossRef]
- Liu, L.-G. High pressure NaAlSiO4: The first silicate calcium ferrite isotype. Geophys. Res. Lett. 1977, 4, 183–186. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Lv, C.; Su, X.; Liu, Y.; Tang, R.; Chen, J.; Hu, Q.; Mao, H.-K.; Mao, W.L. Evidence for oxygenation of Fe-Mg oxides at mid-mantle conditions and the rise of deep oxygen. Natl. Sci. Rev. 2020, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lord, O.T.; Wann, E.T.H.; Hunt, S.A.; Walker, A.M.; Santangeli, J.; Walter, M.J.; Dobson, D.P.; Wood, I.G.; Vočadlo, L.; Morard, G.; et al. The NiSi melting curve to 70GPa. Phys. Earth Planet. Inter. 2014, 233, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Lord, O.T.; Wood, I.G.; Dobson, D.P.; Vočadlo, L.; Wang, W.; Thomson, A.R.; Wann, E.T.H.; Morard, G.; Mezouar, M.; Walter, M.J. The melting curve of Ni to 1 Mbar. Earth Planet. Sci. Lett. 2014, 408, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Shen, G.; Mao, H.K. Double-sided laser heating system at HPCAT for in situ x-ray diffraction at high pressures and high temperatures. J. Phys. Condens. Matter 2006, 18, S1097–S1103. [Google Scholar] [CrossRef]
- Ming, L.c.; Bassett, W.A. Laser heating in the diamond anvil press up to 2000°C sustained and 3000°C pulsed at pressures up to 260 kilobars. Rev. Sci. Instrum. 1974, 45, 1115–1118. [Google Scholar] [CrossRef]
- Miyagi, L.; Kanitpanyacharoen, W.; Raju, S.V.; Kaercher, P.; Knight, J.; MacDowell, A.; Wenk, H.-R.; Williams, Q.; Alarcon, E.Z. Combined resistive and laser heating technique for in situ radial X-ray diffraction in the diamond anvil cell at high pressure and temperature. Rev. Sci. Instrum. 2013, 84, 025118. [Google Scholar] [CrossRef] [Green Version]
- Nabiei, F.; Badro, J.; Boukaré, C.; Hébert, C.; Cantoni, M.; Borensztajn, S.; Wehr, N.; Gillet, P. Investigating Magma Ocean Solidification on Earth Through Laser-Heated Diamond Anvil Cell Experiments. Geophys. Res. Lett. 2021, 48, e2021GL092446. [Google Scholar] [CrossRef]
- Ohfuji, H.; Irifune, T.; Okada, T.; Yagi, T.; Sumiya, H. Laser heating in nano-polycrystalline diamond anvil cell. J. Phys. Conf. Ser. 2010, 215, 5. [Google Scholar] [CrossRef]
- Ohtaka, O.; Andrault, D.; Bouvier, P.; Schultz, E.; Mezouar, M. Phase relations and equation of state of ZrO2 to 100 GPa. J. Appl. Crystallogr. 2005, 38, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Panero, W.R.; Jeanloz, R. Temperature gradients in the laser-heated diamond anvil cell. J. Geophys. Res. Solid Earth 2001, 106, 6493–6498. [Google Scholar] [CrossRef]
- Santamaría-Pérez, D.; Boehler, R. FeSi melting curve up to 70 GPa. Earth Planet. Sci. Lett. 2008, 265, 743–747. [Google Scholar] [CrossRef]
- Pigott, J.S.; Reaman, D.M.; Panero, W.R. Microfabrication of controlled-geometry samples for the laser-heated diamond-anvil cell using focused ion beam technology. Rev. Sci. Instrum. 2011, 82, 115106. [Google Scholar] [CrossRef] [PubMed]
- Pigott, J.S.; Velisavljevic, N.; Moss, E.K.; Draganic, N.; Jacobsen, M.K.; Meng, Y.; Hrubiak, R.; Sturtevant, B.T. Experimental melting curve of zirconium metal to 37 GPa. J. Phys. Condens. Matter 2020, 32, 355402. [Google Scholar] [CrossRef]
- Polvani, D.A.; Meng, J.F.; Hasegawa, M.; Badding, J.V. Measurement of the thermoelectric power of very small samples at ambient and high pressures. Rev. Sci. Instrum. 1999, 70, 3586–3589. [Google Scholar] [CrossRef]
- Prakapenka, V.B.; Kubo, A.; Kuznetsov, A.; Laskin, A.; Shkurikhin, O.; Dera, P.; Rivers, M.L.; Sutton, S.R. Advanced flat top laser heating system for high pressure research at GSECARS: Application to the melting behavior of germanium. High Press. Res. 2008, 28, 225–235. [Google Scholar] [CrossRef]
- Prakapenka, V.B.; Shen, G.; Dubrovinsky, L.S. Carbon transport in diamond anvil cells. High Temp.-High Press. 2003, 35/36, 237–249. [Google Scholar] [CrossRef]
- Raju, S.V.; Hrubiak, R.; Drozd, V.; Saxena, S. Laser-assisted processing of Ni-Al-Co-Ti under high pressure. Mater. Manuf. Process. 2017, 32, 1606–1611. [Google Scholar] [CrossRef]
- Ross, M.; Boehler, R.; Söderlind, P. Xenon Melting Curve to 80 GPa and 5p-d Hybridization. Phys. Rev. Lett. 2005, 95, 257801. [Google Scholar] [CrossRef]
- Runge, C.E.; Kubo, A.; Kiefer, B.; Meng, Y.; Prakapenka, V.B.; Shen, G.; Cava, R.J.; Duffy, T.S. Equation of state of MgGeO3 perovskite to 65 GPa: Comparison with the post-perovskite phase. Phys. Chem. Miner. 2006, 33, 699–709. [Google Scholar] [CrossRef]
- Sadovyi, B.; Wierzbowska, M.; Stelmakh, S.; Boccato, S.; Gierlotka, S.; Irifune, T.; Porowski, S.; Grzegory, I. Experimental and theoretical evidence of the temperature-induced wurtzite to rocksalt phase transition in GaN under high pressure. Phys. Rev. B Condens. Matter 2020, 102, 235109. [Google Scholar] [CrossRef]
- Saha, P.; Mukherjee, G.D. Temperature measurement in double-sided laser-heated diamond anvil cell and reaction of carbon. Indian J. Phys. 2021, 95, 621–628. [Google Scholar] [CrossRef]
- Saha, P.; Mazumder, A.; Mukherjee, G.D. Thermal conductivity of dense hcp iron: Direct measurements using laser heated diamond anvil cell. Geosci. Front. 2020, 11, 1755–1761. [Google Scholar] [CrossRef]
- Salem, R.; Matityahu, S.; Melchior, A.; Nikolaevsky, M.; Noked, O.; Sterer, E. Image analysis of speckle patterns as a probe of melting transitions in laser-heated diamond anvil cell experiments. Rev. Sci. Instrum. 2015, 86, 093907. [Google Scholar] [CrossRef] [Green Version]
- Santamaría-Pérez, D.; Ross, M.; Errandonea, D.; Mukherjee, G.D.; Mezouar, M.; Boehler, R. X-ray diffraction measurements of Mo melting to 119 GPa and the high pressure phase diagram. J. Chem. Phys. 2009, 130, 124509. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.K.; Dubrovinsky, L.S.; Häggkvist, P.; Cerenius, Y.; Shen, G.; Mao, H.K. Synchrotron X-Ray Study of Iron at High Pressure and Temperature. Science 1995, 269, 1703–1704. [Google Scholar] [CrossRef]
- Schultz, E.; Mezouar, M.; Crichton, W.; Bauchau, S.; Blattmann, G.; Andrault, D.; Fiquet, G.; Boehler, R.; Rambert, N.; Sitaud, B.; et al. Double-sided laser heating system for in situ high pressure–high temperature monochromatic X-ray diffraction at the esrf. High Press. Res. 2005, 25, 71–83. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.-K.; Hemley, R.J.; Duffy, T.S.; Rivers, M.L. Melting and crystal structure of iron at high pressures and temperatures. Geophys. Res. Lett. 1998, 25, 373–376. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.; Rivers, M.L.; Wang, Y.; Sutton, S.R. Laser heated diamond cell system at the Advanced Photon Source for in situ X-ray measurements at high pressure and temperature. Rev. Sci. Instrum. 2001, 72, 1273–1282. [Google Scholar] [CrossRef]
- Shen, G.; Prakapenka, V.B.; Rivers, M.L.; Sutton, S.R. Structure of Liquid Iron at Pressures up to 58 GPa. Phys. Rev. Lett. 2004, 92, 185701. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.-k.; Hemley, R. Laser-Heated Diamond Anvil Cell Technique: Double-Sided Heating with Multimode Nd:YAG Laser. In Advanced Materials ‘96 New Trends in High Pressure Research; Akaishi, M., Ed.; The Institute: Tsukuba, Japan, 1996. [Google Scholar]
- Shieh, S.R.; Duffy, T.S.; Shen, G. X-ray diffraction study of phase stability in SiO2 at deep mantle conditions. Earth Planet. Sci. Lett. 2005, 235, 273–282. [Google Scholar] [CrossRef]
- Singh, A.K.; Andrault, D.; Bouvier, P. X-ray diffraction from stishovite under nonhydrostatic compression to 70GPa: Strength and elasticity across the tetragonal→orthorhombic transition. Phys. Earth Planet. Inter. 2012, 208–209, 1–10. [Google Scholar] [CrossRef]
- Sinmyo, R.; Hirose, K. The Soret diffusion in laser-heated diamond-anvil cell. Phys. Earth Planet. Inter. 2010, 180, 172–178. [Google Scholar] [CrossRef]
- Sinmyo, R.; Pesce, G.; Greenberg, E.; McCammon, C.; Dubrovinsky, L. Lower mantle electrical conductivity based on measurements of Al, Fe-bearing perovskite under lower mantle conditions. Earth Planet. Sci. Lett. 2014, 393, 165–172. [Google Scholar] [CrossRef]
- Spiekermann, G.; Kupenko, I.; Petitgirard, S.; Harder, M.; Nyrow, A.; Weis, C.; Albers, C.; Biedermann, N.; Libon, L.; Sahle, C.J.; et al. A portable on-axis laser-heating system for near-90degrees X-ray spectroscopy: Application to ferropericlase and iron silicide. J. Synchrotron Radiat. 2020, 27, 414–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutzmann, V.; Dewaele, A.; Bouchet, J.; Bottin, F.; Mezouar, M. High-pressure melting curve of titanium. Phys. Rev. B Condens. Matter. 2015, 92, 224110. [Google Scholar] [CrossRef]
- Auzende, A.-L.; Gillot, J.; Coquet, A.; Hennet, L.; Ona-Nguema, G.; Bonnin, D.; Esteve, I.; Roskosz, M.; Fiquet, G. Synthesis of amorphous MgO-rich peridotitic starting material for laser-heated diamond anvil cell experiments—Application to iron partitioning in the mantle. High Press. Res. 2011, 31, 199–213. [Google Scholar] [CrossRef]
- Tateno, S.; Hirose, K.; Ohishi, Y.; Tatsumi, Y. The Structure of Iron in Earth’s Inner Core. Science 2010, 330, 359–361. [Google Scholar] [CrossRef]
- Watanuki, T.; Shimomura, O.; Yagi, T.; Kondo, T.; Isshiki, M. Construction of laser-heated diamond anvil cell system for in situ x-ray diffraction study at SPring-8. Rev. Sci. Instrum. 2001, 72, 1289–1292. [Google Scholar] [CrossRef]
- Weck, G.; Recoules, V.; Queyroux, J.-A.; Datchi, F.; Bouchet, J.; Ninet, S.; Garbarino, G.; Mezouar, M.; Loubeyre, P. Determination of the melting curve of gold up to 110 GPa. Phys. Rev. B Condens. Matter. 2020, 101, 014106. [Google Scholar] [CrossRef] [Green Version]
- Yagi, T.; Susaki, J.-I. A Laser Heating System for Diamond Anvil Using CO2. In High-Pressure Research: Application to Earth and Planetary Sciences; Terra Scientific Publishing Company: Tokyo, Japan, 1992; pp. 51–54. [Google Scholar]
- Yang, L.; Karandikar, A.; Boehler, R. Flash heating in the diamond cell: Melting curve of rhenium. Rev. Sci. Instrum. 2012, 83, 063905. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.S.; Akella, J.; Moriarty, J.A. High-pressure melting temperatures of uranium: Laser-heating experiments and theoretical calculations. Phys. Rev. B Condens. Matter. 1993, 48, 15529–15534. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.S.; Akella, J.; Campbell, A.J.; Mao, H.K.; Hemley, R.J. Phase Diagram of Iron by in Situ X-ray Diffraction: Implications for Earth’s Core. Science 1995, 270, 1473–1475. [Google Scholar] [CrossRef]
- Yoo, C.S.; Söderlind, P.; Moriarty, J.A.; Cambell, A.J. dhcp as a possible new ϵ′ phase of iron at high pressures and temperatures. Phys. Lett. A 1996, 214, 65–70. [Google Scholar] [CrossRef]
- Yusa, H.; Takemura, K.; Matsui, Y.; Morishima, H.; Watanabe, K.; Yamawaki, H.; Aoki, K. Direct transformation of graphite to cubic diamond observed in a laser-heated diamond anvil cell. Appl. Phys. Lett. 1998, 72, 1843–1845. [Google Scholar] [CrossRef]
- Zerr, A.; Boehier, R. Melting of (Mg, Fe)SiO3-Perovskite to 625 Kilobars: Indication of a High Melting Temperature in the Lower Mantle. Science 1993, 262, 553–555. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Serghiou, G.; Schwarz, M.; Kroke, E.; Riedel, R.; Fueß, H.; Kroll, P.; Boehler, R. Synthesis of cubic silicon nitride. Nature 1999, 400, 340–342. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Mao, H.-k. Unit cell determination of coexisting post-perovskite and H-phase in (Mg,Fe)SiO3 using multigrain XRD: Compositional variation across a laser heating spot at 119 GPa. Prog. Earth Planet. Sci. 2016, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Jackson, J.M.; Zhao, J.; Sturhahn, W.; Alp, E.E.; Hu, M.Y.; Toellner, T.S.; Murphy, C.A.; Prakapenka, V.B. Temperature of Earth’s core constrained from melting of Fe and Fe0.9Ni0.1 at high pressures. Earth Planet. Sci. Lett. 2016, 447, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Zinin, P.V.; Prakapenka, V.B.; Burgess, K.; Odake, S.; Chigarev, N.; Sharma, S.K. Combined laser ultrasonics, laser heating, and Raman scattering in diamond anvil cell system. Rev. Sci. Instrum. 2016, 87, 123908. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Ma, Y.; Mao, H.-K.; Hemley, R.J.; Gramsch, S.A. A diamond gasket for the laser-heated diamond anvil cell. Rev. Sci. Instrum. 2001, 72, 1298–1301. [Google Scholar] [CrossRef]
- Weathers, M.S.; Bassett, W.A. Melting of carbon at 50 to 300 kbar. Phys. Chem. Miner. 1987, 15, 105–112. [Google Scholar] [CrossRef]
- Huang, D.; Siebert, J.; Badro, J. High pressure partitioning behavior of Mo and W and late sulfur delivery during Earth’s core formation. Geochim. Cosmochim. Acta 2021, 310, 19–31. [Google Scholar] [CrossRef]
- Kurnosov, A.; Marquardt, H.; Dubrovinsky, L.; Potapkin, V. A waveguide-based flexible CO2-laser heating system for diamond-anvil cell applications. C.R. Geosci. 2019, 351, 280–285. [Google Scholar] [CrossRef]
- Chidester, B.A.; Thompson, E.C.; Fischer, R.A.; Heinz, D.L.; Prakapenka, V.B.; Meng, Y.; Campbell, A.J. Experimental thermal equation of state of B2−KCl. Phys. Rev. B Condens. Matter. 2021, 104, 094107. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Geng, H.Y.; Salke, N.P.; Gao, Z.; Li, J.; Sekine, T.; Wang, Q.; Greenberg, E.; Prakapenka, V.B.; et al. Melting curve of vanadium up to 256 GPa: Consistency between experiments and theory. Phys. Rev. B Condens. Matter. 2020, 102, 214104. [Google Scholar] [CrossRef]
- Gaida, N.A.; Niwa, K.; Sasaki, T.; Hasegawa, M. Phase relations and thermoelasticity of magnesium silicide at high pressure and temperature. J. Chem. Phys. 2021, 154, 144701. [Google Scholar] [CrossRef]
- Anzellini, S.; Burakovsky, L.; Turnbull, R.; Bandiello, E.; Errandonea, D. P–V–T Equation of State of Iridium Up to 80 GPa and 3100 K. Crystals 2021, 11, 452. [Google Scholar] [CrossRef]
- Liu, L.-g. Silicate perovskite from phase transformations of pyrope-garnet at high pressure and temperature. Geophys. Res. Lett. 1974, 1, 277–280. [Google Scholar] [CrossRef]
- Nishiyama, N.; Langer, J.; Sakai, T.; Kojima, Y.; Holzheid, A.; Gaida, N.A.; Kulik, E.; Hirao, N.; Kawaguchi, S.I.; Irifune, T.; et al. Phase relations in silicon and germanium nitrides up to 98 GPa and 2400 °C. J. Am. Ceram. Soc. 2019, 102, 2195–2202. [Google Scholar] [CrossRef]
- Bassett, W.A.; Li-Chung, M. Disproportionation of Fe2SiO4 to 2FeO+SiO2 at pressures up to 250kbar and temperatures up to 3000 °C. Phys. Earth Planet. Inter. 1972, 6, 154–160. [Google Scholar] [CrossRef]
- Kavner, A.; Jeanloz, R. The high-pressure melting curve of Allende meteorite. Geophys. Res. Lett. 1998, 25, 4161–4164. [Google Scholar] [CrossRef]
- Boehler, R.; von Bargen, N.; Chopelas, A. Melting, thermal expansion, and phase transitions of iron at high pressures. J. Geophys. Res. Solid Earth 1990, 95, 21731–21736. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Ohta, K.; Yagi, T.; Hirose, K.; Ohishi, Y. Measurements of sound velocity in iron–nickel alloys by femtosecond laser pulses in a diamond anvil cell. Phys. Chem. Miner. 2018, 45, 589–595. [Google Scholar] [CrossRef]
- Deemyad, S.; Sterer, E.; Barthel, C.; Rekhi, S.; Tempere, J.; Silvera, I.F. Pulsed laser heating and temperature determination in a diamond anvil cell. Rev. Sci. Instrum. 2005, 76, 125104. [Google Scholar] [CrossRef]
- Deemyad, S.; Silvera, I.F. Melting Line of Hydrogen at High Pressures. Phys. Rev. Lett. 2008, 100, 155701. [Google Scholar] [CrossRef] [Green Version]
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A.; Dzivenko, D.A.; Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 2004, 3, 558–563. [Google Scholar] [CrossRef]
- Brygoo, S.; Loubeyre, P.; Millot, M.; Rygg, J.R.; Celliers, P.M.; Eggert, J.H.; Jeanloz, R.; Collins, G.W. Evidence of hydrogen−helium immiscibility at Jupiter-interior conditions. Nature 2021, 593, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Nissim, N.; Eliezer, S.; Werdiger, M. The sound velocity throughout the P-ρ phase-space with application to laser induced shock wave in matter precompressed by a diamond anvil cell. J. Appl. Phys. 2014, 115, 213503. [Google Scholar] [CrossRef]
- Brygoo, S.; Millot, M.; Loubeyre, P.; Lazicki, A.E.; Hamel, S.; Qi, T.; Celliers, P.M.; Coppari, F.; Eggert, J.H.; Fratanduono, D.E.; et al. Analysis of laser shock experiments on precompressed samples using a quartz reference and application to warm dense hydrogen and helium. J. Appl. Phys. 2015, 118, 195901. [Google Scholar] [CrossRef]
- Loubeyre, P.; Celliers, P.M.; Hicks, D.G.; Henry, E.; Dewaele, A.; Pasley, J.; Eggert, J.; Koenig, M.; Occelli, F.; Lee, K.M.; et al. Coupling static and dynamic compressions: First measurements in dense hydrogen. High Press. Res. 2004, 24, 25–31. [Google Scholar] [CrossRef]
- Loubeyre, P.; Brygoo, S.; Eggert, J.; Celliers, P.M.; Spaulding, D.K.; Rygg, J.R.; Boehly, T.R.; Collins, G.W.; Jeanloz, R. Extended data set for the equation of state of warm dense hydrogen isotopes. Phys. Rev. B Condens. Matter. 2012, 86, 144115. [Google Scholar] [CrossRef]
- Nissim, N.; Eliezer, S.; Werdiger, M.; Perelmutter, L. Approaching the “cold curve” in laser-driven shock wave experiment of a matter precompressed by a partially perforated diamond anvil. Laser Part. Beams 2013, 31, 73–79. [Google Scholar] [CrossRef]
- Coppari, F.; Smith, R.F.; Eggert, J.H.; Wang, J.; Rygg, J.R.; Lazicki, A.; Hawreliak, J.A.; Collins, G.W.; Duffy, T.S. Experimental evidence for a phase transition in magnesium oxide at exoplanet pressures. Nat. Geosci. 2013, 6, 926–929. [Google Scholar] [CrossRef]
- Vance, S.; Harnmeijer, J.; Kimura, J.; Hussmann, H.; deMartin, B.; Brown, J.M. Hydrothermal Systems in Small Ocean Planets. Astrobiology 2007, 7, 987–1005. [Google Scholar] [CrossRef]
- Helled, R.; Anderson, J.D.; Podolak, M.; Schubert, G. Interior Models of Uranus and Neptune. Astrophys. J. 2010, 726, 15. [Google Scholar] [CrossRef] [Green Version]
- Faure, G.; Mensing, T. Introduction to Planetary Science; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Guillot, T.; Gautier, D. 10.13—Giant Planets. In Treatise on Geophysics; Schubert, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 439–464. [Google Scholar]
- Walker, D.; Lord, O.T.; Walter, M.J.; Clark, S.M. X-ray absorption contrast images of binary chemical reactions. Chem. Geol. 2009, 260, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Ladlow, M.; Legge, C.H.; Neudeck, T.; Pipe, A.J.; Sheppard, T.; Yang, L.L. Wavelength dependent photo-controlled differential release of compounds from solid phase resin. Chem. Commun. 2003, 2048–2049. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K. Lasers in Materials Processing and Synthesis. In Handbook on Synthesis Strategies for Advanced Materials: Volume-II: Processing and Functionalization of Materials; Tyagi, A.K., Ningthoujam, R.S., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 791–831. [Google Scholar]
- Andrei, F.; Tiliakos, A.; Scarisoreanu, M.; Scarisoreanu, N.D. Advanced Laser Methods for Synthesizing Photocatalysts. In Green Photocatalytic Semiconductors: Recent Advances and Applications; Garg, S., Chandra, A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 399–444. [Google Scholar]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.J.; Shwetharani, R.; Kim, H.-S.; Pasha, S.K.K.; Ashokkumar, M.; Choi, M.Y. Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo- and electrocatalytic applications. Light Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef]
- Yusa, H. Laser-heated diamond anvil cell system for photochemical reaction measurements. Rev. Sci. Instrum. 2001, 72, 1309. [Google Scholar] [CrossRef]
- Ishida, Y.; Iwasaki, N.; Asaumi, K.; Yajima, T.; Maruyama, Y. Tunable picosecond pulses from a short-cavity dye laser under ultra-high pressure using diamond-anvil cell. Appl. Phys. B 1985, 38, 159–163. [Google Scholar] [CrossRef]
- Mao, H.K.; Bell, P.M.; Shaner, J.W.; Steinberg, D.J. Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence pressure gauge from 0.06 to 1 Mbar. J. Appl. Phys. 1978, 49, 3276–3283. [Google Scholar] [CrossRef]
- Bassett, W.A. The birth and development of laser heating in diamond anvil cells. Rev. Sci. Instrum. 2001, 72, 1270–1272. [Google Scholar] [CrossRef]
- Hernandez, H. Collision Energy between Maxwell-Boltzmann Molecules: An Alternative Derivation of Arrhenius Equation. ForsChem Res. 2019. [Google Scholar] [CrossRef]
- Akhtar, M. Synthesis and fundamental property studies of energy material under high pressure. Ph.D. Thesis, University of Louisville, Louisville, KY, USA, 2017. [Google Scholar]
- Spooner, J.; Wiebe, H.; Louwerse, M.; Reader, B.; Weinberg, N. Theoretical analysis of high-pressure effects on conformational equilibria. Can. J. Chem. 2018, 96, 178–189. [Google Scholar] [CrossRef] [Green Version]
- Daviau, K.; Meng, Y.; Lee, K.K.M. SiO2-SiC Mixtures at High Pressures and Temperatures: Implications for Planetary Bodies Containing SiC. J. Geophys. Res. Planets 2019, 124, 2294–2305. [Google Scholar] [CrossRef]
- Arora, A.K.; Sakuntala, T. Decomposition of binary sulphate KHSO4 at high pressure. High Press. Res. 2000, 17, 1–11. [Google Scholar] [CrossRef]
- Kuznetsov, A.Y.; Pereira, A.S.; Shiryaev, A.A.; Haines, J.; Dubrovinsky, L.; Dmitriev, V.; Pattison, P.; Guignot, N. Pressure-Induced Chemical Decomposition and Structural Changes of Boric Acid. J. Phys. Chem. A 2006, 110, 13858–13865. [Google Scholar] [CrossRef]
- Greaves, R.J.; Schlecht, K.D. Gibbs free energy: The criteria for spontaneity. J. Chem. Educ. 1992, 69, 417. [Google Scholar] [CrossRef]
- Demazeau, G. High Pressure and Chemical Bonding in Materials Chemistry. Z. Naturforsch. B 2006, 61, 799–807. [Google Scholar] [CrossRef]
- Cai, W.; Katrusiak, A. Giant negative linear compression positively coupled to massive thermal expansion in a metal–organic framework. Nat. Commun. 2014, 5, 4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, N.; Shekar, N.V.C.; Kumar, N.R.S.; Sahu, P.C. Development of laser-heated diamond anvil cell facility for synthesis of novel materials. Curr. Sci. 2006, 91, 175–182. [Google Scholar]
- Shekar, N.V.C.; Takemura, K.; Yusa, H. Synthesis experiments on in-Sb and B-Sb systems in a laser heated diamond-anvil cell. High Press. Res. 1997, 15, 393–398. [Google Scholar] [CrossRef]
- Kadobayashi, H.; Ohnishi, S.; Ohfuji, H.; Yamamoto, Y.; Muraoka, M.; Yoshida, S.; Hirao, N.; Kawaguchi-Imada, S.; Hirai, H. Diamond formation from methane hydrate under the internal conditions of giant icy planets. Sci. Rep. 2021, 11, 8165. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Kinetics In Solids. Annu. Rev. Phys. Chem. 1997, 48, 125–149. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kinetic effects of pressure on decomposition of solids. Int. Rev. Phys. Chem. 2020, 39, 35–66. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4U, 226–248. [Google Scholar] [CrossRef] [Green Version]
- Laidler, K.J.; King, M.C. Development of transition-state theory. J. Phys. Chem. 1983, 87, 2657–2664. [Google Scholar] [CrossRef]
- Haring, M.M. The Theory of Rate Processes (Glasstone, Samuel; Laidler, Keith J.; Eyring, Henry). J. Chem. Educ. 1942, 19, 249. [Google Scholar] [CrossRef] [Green Version]
- Eyring, H. The Activated Complex in Chemical Reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Asano, T.; Le Noble, W.J. Activation and reaction volumes in solution. Chem. Rev. 1978, 78, 407–489. [Google Scholar] [CrossRef]
- Kornilov, D.A.; Kiselev, V.D. Activation and Reaction Volumes and Their Correlations with the Entropy and Enthalpy Parameters. J. Chem. Eng. Data 2015, 60, 3571–3580. [Google Scholar] [CrossRef]
- Brosh, E.; Shneck, R.Z.; Makov, G. Explicit Gibbs free energy equation of state for solids. J. Phys. Chem. Solids 2008, 69, 1912–1922. [Google Scholar] [CrossRef]
- Lin, W.; Murphy, C.J. A Demonstration of Le Chatelier’s Principle on the Nanoscale. ACS Cent. Sci. 2017, 3, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Chatelier, H. Recherches Expérimentales et Théoriques sur les Équilibres Chimiques; Dunod: Malakoff, France, 1888. [Google Scholar]
- Laidler, K.J. Chemical kinetics, 3rd ed.; Harper Collins: New York, NY, USA, 1987. [Google Scholar]
- Kyaw, K.; Kubota, M.; Watanabe, F.; Matsuda, H.; Hasatani, M. Study of Carbonation of CaO for High Temperature Thermal Energy Storage. J. Chem. Eng. Jpn. 1998, 31, 281–284. [Google Scholar] [CrossRef]
- Benton, A.F.; Drake, L.C. Kinetics of Reaction and Adsorption in the System Silver—Oxygen. J. Am. Chem. Soc. 1934, 56, 255–263. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Vyazovkin, S. Delving into the Kinetics of Reversible Thermal Decomposition of Solids Measured on Heating and Cooling. J. Phys. Chem. C 2017, 121, 15392–15401. [Google Scholar] [CrossRef]
- Laniel, D.; Bykov, M.; Fedotenko, T.; Ponomareva, A.V.; Abrikosov, I.A.; Glazyrin, K.; Svitlyk, V.; Dubrovinsky, L.; Dubrovinskaia, N. High Pressure Investigation of the S–N2 System up to the Megabar Range: Synthesis and Characterization of the SN2 Solid. Inorg. Chem. 2019, 58, 9195–9204. [Google Scholar] [CrossRef] [PubMed]
- Mattesini, M.; de Almeida, J.S.; Dubrovinsky, L.; Dubrovinskaia, N.; Johansson, B.; Ahuja, R. High-pressure and high-temperature synthesis of the cubic TiO2 polymorph. Phys. Rev. B 2004, 70, 212101. [Google Scholar] [CrossRef]
- Lobanov, S.S.; Zhu, Q.; Holtgrewe, N.; Prescher, C.; Prakapenka, V.B.; Oganov, A.R.; Goncharov, A.F. Stable magnesium peroxide at high pressure. Sci. Rep. 2015, 5, 13582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Oganov, A.R.; Zhu, Q.; Lobanov, S.S.; Stavrou, E.; Goncharov, A.F. Stability of numerous novel potassium chlorides at high pressure. Sci. Rep. 2016, 6, 26265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurlo, A.; Dzivenko, D.; Kroll, P.; Riedel, R. High-pressure high-temperature synthesis of Rh2O3-II-type In2O3 polymorph. Phys. Stat. Sol. 2008, 2, 269–271. [Google Scholar] [CrossRef]
- Soignard, E.; Machon, D.; McMillan, P.F.; Dong, J.; Xu, B.; Leinenweber, K. Spinel-Structured Gallium Oxynitride (Ga3O3N) Synthesis and Characterization: An Experimental and Theoretical Study. Chem. Mater. 2005, 17, 5465–5472. [Google Scholar] [CrossRef]
- Clarke, S.M.; Amsler, M.; Walsh, J.P.S.; Yu, T.; Wang, Y.; Meng, Y.; Jacobsen, S.D.; Wolverton, C.; Freedman, D.E. Creating Binary Cu–Bi Compounds via High-Pressure Synthesis: A Combined Experimental and Theoretical Study. Chem. Mater. 2017, 29, 5276–5285. [Google Scholar] [CrossRef]
- Yadav, R.R.; Kumar, L.K. One-dimensional spatially dependent solute transport in semi-infinite porous media: An analytical solution. Int. J. Eng. Sci. Technol. 2017, 9, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Jacob, A.C.; Zeidler, M.D. Self-diffusion in binary mixtures: The system benzene/cyclohexane at high pressures. Phys. Chem. Chem. Phys. 2003, 5, 538–542. [Google Scholar] [CrossRef]
- Noguchi, N.; Okuchi, T. Self-diffusion of protons in H2O ice VII at high pressures: Anomaly around 10 GPa. J. Chem. Phys. 2016, 144, 234503. [Google Scholar] [CrossRef]
- Smoluchowski, M.V. Zur kinetischen Theorie der Transpiration und Diffusion verdünnter Gase. Ann. Phys. 1910, 338, 1559–1570. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.A.; Rodrigo, M. A note on Smoluchowski’s equations with diffusion. Appl. Math. Lett. 2005, 18, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Calef, D.F.; Deutch, J.M. Diffusion-Controlled Reactions. Annu. Rev. Phys. Chem. 1983, 34, 493–524. [Google Scholar] [CrossRef]
- Ehrlich, D.J.; Tsao, J.Y.; Tsao, J.Y. Laser Microfabrication: Thin Film Processes and Lithography; Elsevier Science: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Maxwell, J.L.; Webb, N.; Bradshaw, D.; Black, M.R.; Maskaly, K.; Chavez, C.A.; Espinoza, M.; Vessard, S.; Art, B.; Johnson, S.; et al. On “how to start a fire”, or transverse forced-convection, hyperbaric laser chemical vapor deposition of fibers and textiles. Text. Res. J. 2014, 84, 1976–1986. [Google Scholar] [CrossRef]
- Wang, R.; Chen, W.; Zhang, L.; Liu, D.; Li, X.; Du, Y.; Jin, Z. Diffusivities and atomic mobilities in the Al–Ce–Ni melts. J. Non-Cryst. Solids 2013, 379, 201–207. [Google Scholar] [CrossRef]
- Sekido, N.; Hoshino, A.; Fukuzaki, M.; Maruko, T.; Yamabe-Mitarai, Y. Interdiffusion in the Ir-Rich Solid Solutions of Ir-Pt, Ir-Rh, and Ir-Re Binary Alloys. J. Ph. Equilibria Diffus. 2011, 32, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Emami-Meybodi, H. Apparent diffusion coefficient for adsorption-controlled gas transport in nanoporous media. Chem. Eng. J. 2022, 450, 138105. [Google Scholar] [CrossRef]
- Nachtrieb, N.H.; Resing, H.A.; Rice, S.A. Effect of Pressure on Self-Diffusion in Lead. J. Chem. Phys. 1959, 31, 135–138. [Google Scholar] [CrossRef]
- Jamieson, J.C.; Lawson, A.W.; Nachtrieb, N.D. New Device for Obtaining X-Ray Diffraction Patterns from Substances Exposed to High Pressure. Rev. Sci. Instrum. 1959, 30, 1016–1019. [Google Scholar] [CrossRef]
- Gréaux, S.; Gautron, L.; Andrault, D.; Bolfan-Casanova, N.; Guignot, N.; Bouhifd, M.A. Experimental high pressure and high temperature study of the incorporation of uranium in Al-rich CaSiO3 perovskite. Phys. Earth Planet. Inter. 2009, 174, 254–263. [Google Scholar] [CrossRef]
- Toki, T.; Bellan, J. Investigation of species-mass diffusion in binary-species boundary layers at high pressure using direct numerical simulations. J. Fluid Mech. 2021, 928, A18. [Google Scholar] [CrossRef]
- Alexandrov, D.V.; Galenko, P.K. Selected mode for rapidly growing needle-like dendrite controlled by heat and mass transport. Acta Mater. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Gomez-Perez, N.; Rodriguez, J.F.; McWilliams, R.S. Finite element modeling of melting and fluid flow in the laser-heated diamond-anvil cell. J. Appl. Phys. 2017, 121, 145904. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.Q.; Nygren, E.; Aziz, M.J. Pressure-enhanced crystallization kinetics of amorphous Si and Ge: Implications for point-defect mechanisms. J. Appl. Phys. 1991, 70, 5323–5345. [Google Scholar] [CrossRef] [Green Version]
- Kolasinski, K.W. Catalytic growth of nanowires: Vapor–liquid–solid, vapor–solid–solid, solution–liquid–solid and solid–liquid–solid growth. Curr. Opin. Solid State Mater. Sci. 2006, 10, 182–191. [Google Scholar] [CrossRef]
- Sarjeant, P.T.; Roy, R. New Glassy and Polymorphic Oxide Phases Using Rapid Quenching Techniques. J. Am. Ceram. Soc. 1967, 50, 500–503. [Google Scholar] [CrossRef]
- Steeb, S. Rapidly Quenched Metals. Elsevier Science: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Goarant, F.; Guyot, F.; Peyronneau, J.; Poirier, J.-P. High-pressure and high-temperature reactions between silicates and liquid iron alloys, in the diamond anvil cell, studied by analytical electron microscopy. J. Geophys. Res. Solid Earth 1992, 97, 4477–4487. [Google Scholar] [CrossRef]
- Kobayashi, A.; Steinberg, M. The Thermal Decomposition of Methane in a Tubular Reactor; UD Department of Energy: Washington, DC, USA, 1992; 20p. [Google Scholar]
- Ashcroft, N.W. Metallic Hydrogen: A High-Temperature Superconductor? Phys. Rev. Lett. 1968, 21, 1748–1749. [Google Scholar] [CrossRef]
- Liu, H.; Naumov, I.I.; Hoffmann, R.; Ashcroft, N.W.; Hemley, R.J. Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressure. Proc. Natl. Acad. Sci. USA 2017, 114, 6990–6995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashcroft, N.W. Hydrogen Dominant Metallic Alloys: High Temperature Superconductors? Phys. Rev. Lett. 2004, 92, 187002. [Google Scholar] [CrossRef] [PubMed]
- Simons Collaboration on the Many Electron Problem; Motta, M.; Genovese, C.; Ma, F.; Cui, Z.-H.; Sawaya, R.; Chan, G.K.-L.; Chepiga, N.; Helms, P.; Jiménez-Hoyos, C.; et al. Ground-State Properties of the Hydrogen Chain: Dimerization, Insulator-to-Metal Transition, and Magnetic Phases. Phys. Rev. X 2020, 10, 031058. [Google Scholar] [CrossRef]
- Salke, N.P.; Davari Esfahani, M.M.; Zhang, Y.; Kruglov, I.A.; Zhou, J.; Wang, Y.; Greenberg, E.; Prakapenka, V.B.; Liu, J.; Oganov, A.R.; et al. Synthesis of clathrate cerium superhydride CeH9 at 80-100 GPa with atomic hydrogen sublattice. Nat. Commun. 2019, 10, 4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struzhkin, V.V.; Kim, D.Y.; Stavrou, E.; Muramatsu, T.; Mao, H.-k.; Pickard, C.J.; Needs, R.J.; Prakapenka, V.B.; Goncharov, A.F. Synthesis of sodium polyhydrides at high pressures. Nat. Commun. 2016, 7, 12267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Semenok, D.V.; Duan, D.; Xie, H.; Chen, W.; Huang, X.; Li, X.; Liu, B.; Oganov, A.R.; Cui, T. Superconducting praseodymium superhydrides. Sci. Adv. 2020, 6, eaax6849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, B.; Juarez-Arellano, E.A.; Friedrich, A.; Bayarjargal, L.; Schröder, F.; Biehler, J.; Milman, V.; Clark, S.M.; Yan, J. In situ synchrotron X-ray diffraction study of the formation of TaB2 from the elements in a laser heated diamond anvil cell. Solid State Sci. 2010, 12, 2059–2064. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Friedrich, A.; Bayarjargal, L.; Morgenroth, W.; Kunz, M.; Milman, V. In situ study of the formation of rhenium borides from the elements at high-(p, T) conditions: Extreme incompressibility of Re7B3 and formation of new phases. Solid State Sci. 2013, 25, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Bykova, E.; Ovsyannikov, S.V.; Bykov, M.; Yin, Y.; Fedotenko, T.; Holz, H.; Gabel, S.; Merle, B.; Chariton, S.; Prakapenka, V.B.; et al. Synthesis, crystal structure, and properties of stoichiometric hard tungsten tetraboride, WB. J. Mater. Chem. A 2022, 10, 20111–20120. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Bayarjargal, L.; Friedrich, A.; Milman, V.; Kammler, D.R.; Clark, S.M.; Yan, J.; Koch-Müller, M.; Schröder, F.; et al. Formation of scandium carbides and scandium oxycarbide from the elements at high-(P, T) conditions. J. Solid State Chem. 2010, 183, 975–983. [Google Scholar] [CrossRef]
- Winkler, B.; Juarez-Arellano, E.A.; Friedrich, A.; Bayarjargal, L.; Yan, J.; Clark, S.M. Reaction of titanium with carbon in a laser heated diamond anvil cell and reevaluation of a proposed pressure-induced structural phase transition of TiC. J. Alloys Compd. 2009, 478, 392–397. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Friedrich, A.; Wilson, D.J.; Koch-Müller, M.; Knorr, K.; Vogel, S.C.; Wall, J.J.; Reiche, H.; Crichton, W.; et al. Reaction of rhenium and carbon at high pressures and temperatures. Z. Kristallogr. Cryst. Mater. 2008, 223, 492–501. [Google Scholar] [CrossRef]
- Bhadram, V.S.; Kim, D.Y.; Strobel, T.A. High-Pressure Synthesis and Characterization of Incompressible Titanium Pernitride. Chem. Mater. 2016, 28, 1616–1620. [Google Scholar] [CrossRef]
- Niwa, K.; Terabe, T.; Kato, D.; Takayama, S.; Kato, M.; Soda, K.; Hasegawa, M. Highly Coordinated Iron and Cobalt Nitrides Synthesized at High Pressures and High Temperatures. Inorg. Chem. 2017, 56, 6410–6418. [Google Scholar] [CrossRef]
- Binns, J.; Donnelly, M.-E.; Peña-Alvarez, M.; Wang, M.; Gregoryanz, E.; Hermann, A.; Dalladay-Simpson, P.; Howie, R.T. Direct Reaction between Copper and Nitrogen at High Pressures and Temperatures. J. Phys. Chem. Lett. 2019, 10, 1109–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, K.; Fukui, R.; Terabe, T.; Kawada, T.; Kato, D.; Sasaki, T.; Soda, K.; Hasegawa, M. High-Pressure Synthesis and Phase Stability of Nickel Pernitride. Eur. J. Inorg. Chem. 2019, 2019, 3753–3757. [Google Scholar] [CrossRef]
- Ravindran, T.R.; Badding, J.V. UV Raman studies on carbon nitride structures. J. Mater. Sci. 2006, 41, 7145–7149. [Google Scholar] [CrossRef]
- Horvath-Bordon, E.; Riedel, R.; McMillan, P.F.; Kroll, P.; Miehe, G.; van Aken, P.A.; Zerr, A.; Hoppe, P.; Shebanova, O.; McLaren, I.; et al. High-Pressure Synthesis of Crystalline Carbon Nitride Imide, C2N2(NH). Angew. Chem. Int. Ed. 2007, 46, 1476–1480. [Google Scholar] [CrossRef]
- Laniel, D.; Weck, G.; Loubeyre, P. Direct Reaction of Nitrogen and Lithium up to 75 GPa: Synthesis of the Li3N, LiN, LiN2, and LiN5 Compounds. Inorg. Chem. 2018, 57, 10685–10693. [Google Scholar] [CrossRef]
- Patel, N.N.; Meenakshi, S.; Sharma, S.M. LHDAC setup for high temperature and high pressure studies. AIP Conf. Proc. 2014, 1591, 664–665. [Google Scholar] [CrossRef]
- Friedrich, A.; Winkler, B.; Bayarjargal, L.; Juarez Arellano, E.A.; Morgenroth, W.; Biehler, J.; Schröder, F.; Yan, J.; Clark, S.M. In situ observation of the reaction of tantalum with nitrogen in a laser heated diamond anvil cell. J. Alloys Compd. 2010, 502, 5–12. [Google Scholar] [CrossRef]
- Åberg, D.; Erhart, P.; Crowhurst, J.; Zaug, J.M.; Goncharov, A.F.; Sadigh, B. Pressure-induced phase transition in the electronic structure of palladium nitride. Phys. Rev. B 2010, 82, 104116. [Google Scholar] [CrossRef] [Green Version]
- Ismailova, L.; Bykova, E.; Bykov, M.; Cerantola, V.; McCammon, C.; Ballaran, T.B.; Bobrov, A.; Sinmyo, R.; Dubrovinskaia, N.; Glazyrin, K.; et al. Stability of Fe,Al-bearing bridgmanite in the lower mantle and synthesis of pure Fe-bridgmanite. Sci. Adv. 2016, 2, e1600427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikosaka, K.; Sinmyo, R.; Hirose, K.; Ishii, T.; Ohishi, Y. The stability of Fe5O6 and Fe4O5 at high pressure and temperature. Am. Mineral. 2019, 104, 1356–1359. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Lv, C.; Zhang, F.; Fu, S.; Prakapenka, V.B.; Wang, C.; Ho, K.; Lin, J.; Wentzcovitch, R.M. Iron-rich Fe–O compounds at Earth’s core pressures. Innovation 2023, 4, 100354. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Worth, N.; Pickard, C.J.; Needs, R.J.; Pascarelli, S.; Mathon, O.; Mezouar, M.; Irifune, T. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure. Nat. Chem. 2016, 8, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Kim, D.Y.; Liu, J.; Meng, Y.; Yang, L.; Zhang, D.; Mao, W.L.; Mao, H.-K. Dehydrogenation of goethite in Earth’s deep lower mantle. Proc. Natl. Acad. Sci. USA 2017, 114, 1498–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koemets, E.; Fedotenko, T.; Khandarkhaeva, S.; Bykov, M.; Bykova, E.; Thielmann, M.; Chariton, S.; Aprilis, G.; Koemets, I.; Glazyrin, K.; et al. Chemical Stability of FeOOH at High Pressure and Temperature, and Oxygen Recycling in Early Earth History. Eur. J. Inorg. Chem. 2021, 2021, 3048–3053. [Google Scholar] [CrossRef]
- Santoro, M.; Gorelli, F.A.; Bini, R.; Salamat, A.; Garbarino, G.; Levelut, C.; Cambon, O.; Haines, J. Retraction Note: Carbon enters silica forming a cristobalite-type CO2–SiO2 solid solution. Nat. Commun. 2016, 7, 13417. [Google Scholar] [CrossRef] [Green Version]
- Santamaria-Perez, D.; McGuire, C.; Makhluf, A.; Kavner, A.; Chuliá-Jordan, R.; Jorda, J.L.; Rey, F.; Pellicer-Porres, J.; Martinez-García, D.; Rodriguez-Hernández, P.; et al. Correspondence: Strongly-driven Re+CO2 redox reaction at high-pressure and high-temperature. Nat. Commun. 2016, 7, 13647. [Google Scholar] [CrossRef]
- Scott, H.P.; Doczy, V.M.; Frank, M.R.; Hasan, M.; Lin, J.-F.; Yang, J. Magnesite formation from MgO and CO2 at the pressures and temperatures of Earth’s mantle. Am. Mineral. 2013, 98, 1211–1218. [Google Scholar] [CrossRef]
- Nesper, R. Bonding Patterns in Intermetallic Compounds. Angew. Chem. Int. Ed. 1991, 30, 789–817. [Google Scholar] [CrossRef]
- Lin, J.-F.; Gregoryanz, E.; Struzhkin, V.V.; Somayazulu, M.; Mao, H.-K.; Hemley, R.J. Melting behavior of H2O at high pressures and temperatures. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef]
- Sanjay Kumar, N.R.; Chandra Shekar, N.V.; Sahu, P.C. Development of Nd-YAG laser heated diamond anvil cell facility and HPHT synthesis of WGe. AIP Conf. Proc. 2013, 1512, 474–475. [Google Scholar] [CrossRef]
- Yin, Y.; Akbar, F.I.; Bykova, E.; Aslandukova, A.; Laniel, D.; Aslandukov, A.; Bykov, M.; Hanfland, M.; Garbarino, G.; Jia, Z.; et al. Synthesis of rare-earth metal compounds through enhanced reactivity of alkali halides at high pressures. Commun. Chem. 2022, 5, 122. [Google Scholar] [CrossRef] [PubMed]










| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| c-Diamond | a: methane hydrate b: (C2H4) n | a: 13–45 b: 11–29 | a: CO2 [10.6 µm] b: CO2 [10.6 µm] | a: [200 W], 50 μm b: [80 W], 2500–4000 K | a: [252] b: [9] |
| Diamond | a: CH4 b: CH4 | a: 10 to 50 b: 16.8 | a: Nd: YAG [1062 nm] b: Nd: YAG or CO2 | a: CW, 2000 to 3000 K b: CW, >3000 K | a: [48] b: [75] |
| Diamond | CaCO3 | 9–21 | CO2 [10.6 μm] | PW: 8 μs, [10–250 W], 40 μm, 3500 K | [83] |
| Diamond | CO2 | 30–80 | CO2 [10.6 μm] | 100–200 s, [60 W], <30 μm, 300–3000 K | [80] |
| CaCO3 + MgCO3 | (CaMg(CO3)2) | 20–30 | Nd: YAG [1062 nm] | 30 μm,2000 K | [58] |
| C8H12 | C4H6 | 0.6–0.8 | Ar [458/488 nm] | 10–20 mW | [63] |
| C3H8 + H2 | C2H6 | 5 | Nd: YLF | 20–25 µm, 1500 K | [70] |
| CH4 | C2H6, H2 | 5 | Nd: YLF | 20–25 µm, 1500 K | [70] |
| C2H6+ C+ H2 | CH4 | 2 | Nd: YLF | 20–25 µm, 1000–1500 K | [70] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| a: (La,Y)H6 b: (La,Y) H10 | La, Y, NH3BH3 | 170–196 | Data N/A | PW: 100–400 ms, <2000 K | [101] |
| EuH6 and EuH9 | Eu, NH3BH3 | 152 & 170 | YDFL [1050 nm] | 1700 & 2800 K | [6] |
| PdH | Pd, Paraffin oil | 39 | Nd: YAG [1.06 µm] | ≈1500 K | [99] |
| FeH3 | Fe, H2 | 100–125 | Nd: YAG [1.06 µm] | <1500 K | [67] |
| FeH5 | Fe, H2 | 130–140 | Nd: YAG [1.06 µm] | <1500 K | [67] |
| CeH9 | Ce, H2 | 80–100 | Nd: YAG [1.06 µm] | PW: 1 µs, 10 μm, ~2000 K | [302] |
| NaH3 | NaH, H2 | ≥30 | Data N/A | >2000 K | [303] |
| NaH7 | NaH, H2 | 40–50 | Data N/A | >2000 K | [303] |
| PrH9 | Pr, NH3BH3 | 115–130 | Nd: YAG [1.06 µm] | 1650 K | [304] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| LaB8 | 2B + LaB6 | 108 | Nd: YAG [1.06 µm] | PW, ≈2100 K | [18] |
| TaB2 | Ta, B | 14–23.7 | Nd: YLF | [10–15 W], 1600–2000 K | [305] |
| ReB2 | Re, B | 8 | Data N/A | 1500 K | [69] |
| Re7B3 | Re, B | 8.9–22.1 | Data N/A | 120–360 s, [8–25 W], 30 µm, 1500–4000 K | [306] |
| WB4 | W, B | 30.3 | Data N/A | 10 µm, 2300 K | [307] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| Ru2C | Ru, C | 5 | CO2 [10.6 µm] | CW, [120 W], ≈40 µm, 2000 K | [14] |
| PdCx | Pd and C | 52 | Nd: YAG [1.06 µm] | ≈2500 to 3000 K | [99] |
| c-Sc2C3 and c-Sc4C3 | Sc0.87(6)O0.13(6) | 9 | Nd: YLF [1090 nm] | CW, 10–15 W,≈25 μm ≈1600–2200 K | [308] |
| δ-TiC | Ti, graphite | 15 | Nd: YLF [1090 nm] | CW, [<20 W], 1600–2000 K | [309] |
| c-TaC | Ta, graphite | 8.6–14.3 | Data N/A | ≤2300 K | [69] |
| c-Ta2C | Ta, graphite | 8.6–14.3 | Data N/A | ≤2300 K | [69] |
| a: ReCx b: Re2C | Re, graphite | a: 67 b: 20–40 | a: Nd: YAG b: Nd: YLF | a: [80 W], 3800 K b: CW, [10–15 W], 15–30 µm, 1000–2200 K | a: [310] b: [17] |
| PtC | Pt,C | 85 | Nd: YLF, Nd: YAG | ≈2600 K | [57] |
| CrC | Cr, C, RCH2OH | 5–5.3 | CO2 [10.6 µm] | 50 min, [120 W], ~30 μm, 1500 K | [19] |
| ZnS type-MnC | Mn, C | 4.7–9.2 | CO2 [10.6 µm] | [120 W], 30 μm, 2000 K | [7] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| a. c-BN b. h-BN | B, N | a.14.6 b. 2 | Nd: YAG [1.06 µm] | a. ≈30 W, 2300 K b. ≈30 W, ≈1800 K | [96] |
| c-Si3N4 | a: Si, N b: β-Si3N4 | a: 15 b: 30 | a: Nd: YLF [527 nm] b: CO2 [10.6 µm] | a: CW: 1–10 min, [14.5 W], 2200 K b: [≤120 W], ≈2800 K | [200] |
| BP-N | pure N and TiN/Pb + N2 precursors | 130–140 | Nd: YAG [1.06 µm] | ≈15 μm, 2500 K | [22] |
| c-Ge3N4 | Ge, N | 14–20 | Nd: YLF [1.053 µm] | 55 W, 2000 K | [61] |
| Fe3N2 + FeN | Fe, N2 | 49.6 | [1.07 µm] | 1900 K | [65] |
| FeN2 + FeN | Fe, N2 | 58.5–69.6 | [1.07 µm] | 2100–2200 K | [65] |
| FeN4 | FeN2, FeN, N2 | 106–135 | [1.07 µm] | >2000 K | [65] |
| TiN2 | TiN, N2 | 73 | YLF [1.06 µm] | 2400 K | [311] |
| CoN2 | Co, N2 | 39.9 | IR laser | Data N/A | [312] |
| CuN2 | Cu, N2 | >50 | Nd: YAG [1.064 µm] | >1500 | [313] |
| NiN2 | Ni, N2 | ~40 | IR [1.09 µm] | 300 K | [314] |
| C3N4 | C2(CN)4 | 40 | YLF [1.054 µm] | Data N/A | [315] |
| C2N2(NH) | C2N4H4 | 27–42 | YLF [1.054 µm] | ~1950–2500 K | [316] |
| β-Li3N | Li, N2 | 3.5, 25.2 | YLF | ~1500, ~2500 K | [317] |
| LiN2 | Li, N2 | 10.5–73.6 | YLF | ~1500–2500 K | [317] |
| LiN5 | Li, N2 | 73.6 | YLF | ~2500 K | [317] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| GaN | Ga, LN2 | 9 | YDFL [1.07 µm] | CW: 90 min, [17 W], 1925 K | [318] |
| NaCl-typeTiN | Ti, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| IrU2C2-type-Mn3N2 | Mn, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| PbO2-type-Fe2N | Fe, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| CFe2-type-Co2N | Co, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| NaCl-type-VN | V, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| NaCl-type-CrN | Cr, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| O3Re-type-Ni3N | Ni, N2 | ≈10 | Nd: YAG | CW: 30 min, [100 W], ≈1800 K | [71] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| γ-Mo2N | Mo, N | 7 | YDFL [1.07 µm] | 600s, [30 W], 2000 K | [41] |
| c-Hf3N4 | Hf, MN, N2 | 18 | Nd: YAG [1053 nm] | CW: 2–20 min, [55 W], 2800 K | [49] |
| c-Zr3N4 | Zr, N2 | 15.6–18 | Nd: YAG [1053 nm] | CW: 2–20 min [55 W], 2500–3000 K | [49] |
| a: Re3N b:h-Re2N | Re, N2 | a: 13–16 b: 20 | Nd: YLF [1090 nm] | [100 W], ≈25 μm, a: 1600–2400 K b: 2000 K | [62] |
| η-Ta2N3 | Ta, N2 | 11–14.1 | Yb fiber | [8–9 W], 1600–2000 K | [319] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| OsN2 | Os, N2 | 43 | N/A | >2000 K | [53] |
| IrN2 | Ir, N2 | 47–64 | N/A | 1600 K | [52,53] |
| PtN2 | Pt, N2 | 45–50 | N/A | 2000 K | [52,54] |
| PdN2 | Pd, N2 | a: 60 b: >58 | a: Nd: YAG [1053 nm] b: Nd: YAG [1064 nm] | a: CW, [55 W], >1000 K b: CW, 1 µm, 800–900 K | a: [59] b: [320] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| FeO | FeS + Mg2SiO4 | 70, 75 and 130 | Nd: YAG [1.06 µm] | [60 W], ≈2250 K | [296] |
| FeO2 | α-Fe2O3+ O2 | 76–78 | Nd: YAG [1.06 µm] | 1800 K | [55] |
| FeO2 | FeOOH | 92 | Nd: YAG [1.06 µm] | 2050 K | [55] |
| Fe5O7 | α-Fe2O3 + Fe2O3 | 40.7 | [1.07 µm] | m, 1800 K | [64] |
| Fe5O7 + O2 | η-Fe2O3 | 71 | [1.07 µm] | 2700–3000 K | [64] |
| Fe25O32 | Fe3O4 | 80.1 | [1.07 µm] | m, 2950 K | [64] |
| FeO + Fe3O4 | Fe5O6 | 38–59 | Yb fiber | 100 W, 30 μm,1930–2350 K | [322] |
| FeO + h-Fe3O4 | Fe4O5 | 39 | Yb fiber | 100 W, 30 μm, 1860 K | [322] |
| ε–Fe, FenO | Fe, Fe2O3 | 220–260 | Data N/A | 1 s, 20–30 μm 3000–3500 K | [323] |
| Fe-SiO3 | FeO and SiO2 | 45, 110 | Nd: YAG [1.06 µm] | 3100 K | [321] |
| FeSiO3 | (Mg,Fe)SiO3 +Fe | 25–97 | Nd: YLF | ∼50 μm, 2300–3150 K | [66] |
| Xe2O5 | Xe, O2 | ∼77–83 | Data N/A | >2000 K | [324] |
| Xe3O2 | Xe, O2 | ∼97 | Data N/A | >2000 K | [324] |
| FeO2H | Fe2O3, H2O | 133.5 | Nd: YAG | 2000 K | [325] |
| Fe5O7+ η-Fe2O3 | FeOOH | 52 | Data N/A | 1200 K | [326] |
| P-FeO2 | α-FeO2H | 72–132 | Nd: YAG | 1700–2000 K | [325] |
| CO2–SiO2 | SiO2, CO2 | 16–22 | CO2 [10.6 µm] | m, >4000 K | [327] |
| β-ReO2 | CO2, SiO2, Re | 8–48 | [1.07 µm] | m, 1500–2400 K | [30,328] |
| MgCO3 | MgO, CO2 | 5–40 | Nd: YLF [1.05µm] | 1400–1800 K | [329] |
| Product Material(s) | Precursor(s) | Press. [GPa] | Laser Type [nm or µm] | Laser Parameters: Pulse Length/Exposure Time [s], Powers/Energies [W/J], Spots [μm], or Induced Temps. [K] | Refs. |
|---|---|---|---|---|---|
| O2–H2 | H2O | 8.8 | Data N/A | Up to 700 K | [331] |
| H3O+, OH- | H2O | 56 | Nd: YAG [1.06 µm] | 5–20 s, [50 W], 20–30 µm, 1600 K | [56] |
| GeSn | Ge, Sn | 8 | CO2 [10.6 µm] | CW, [≤125 W], ≈30 µm, up to 2000 K | [98] |
| InSb | In, Sb | a: 100, b: 0.2–10 | CO2 [10.6 µm] | a: CO2, 40 µm, ≥ 5000 K b: CW, [10–15 W], 20 μm | a: [250] b: [251] |
| WGe3 | W, Ge | 2.6 | Nd: YAG [1.06 µm] | CW, [30 W], ≈1274 K | [332] |
| FeBi2 | Fe, Bi | 32 | Nd: YLF [1053 nm] | [170 W], 1400 K | [24] |
| Y2ClC+ Y2Cl | Y, NaCl | 41 | Nd: YAG [1.06 µm] | 2000 K | [333] |
| Dy2ClC + DyCl | Dy, NaCl | 40 | Nd: YAG [1.06 µm] | 2000 K | [333] |
| HP-PdF2type-FeCl2 | FeO, KCl | 160 | Nd: YAG [1.06 µm] | 2100 K | [333] |
| CuBi | Cu, Bi | 3.19–4.88 | IR | ~40 µm, ~450 K | [274] |
| Cu11Bi7 | CuBi | 4.16–10 | IR | ~40 µm, ~920 K | [274] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part III: Laser Reactive Synthesis within Diamond Anvil Cells. J. Manuf. Mater. Process. 2023, 7, 57. https://doi.org/10.3390/jmmp7020057
Alabdulkarim ME, Maxwell WD, Thapliyal V, Maxwell JL. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part III: Laser Reactive Synthesis within Diamond Anvil Cells. Journal of Manufacturing and Materials Processing. 2023; 7(2):57. https://doi.org/10.3390/jmmp7020057
Chicago/Turabian StyleAlabdulkarim, Mohamad E., Wendy D. Maxwell, Vibhor Thapliyal, and James L. Maxwell. 2023. "A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part III: Laser Reactive Synthesis within Diamond Anvil Cells" Journal of Manufacturing and Materials Processing 7, no. 2: 57. https://doi.org/10.3390/jmmp7020057
APA StyleAlabdulkarim, M. E., Maxwell, W. D., Thapliyal, V., & Maxwell, J. L. (2023). A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part III: Laser Reactive Synthesis within Diamond Anvil Cells. Journal of Manufacturing and Materials Processing, 7(2), 57. https://doi.org/10.3390/jmmp7020057








