Chemical Composition Effects on the Microstructure and Hot Hardness of NiCrSiFeB Self-Fluxing Alloys Manufactured via Gravity Casting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Casting Process
2.2. Microstructural Analysis
2.3. Phase Identification Using X-ray Diffraction
2.4. Micro- and Nanohardness Measurements of the Phases
2.5. Hot Hardness Tests
3. Results and Discussion
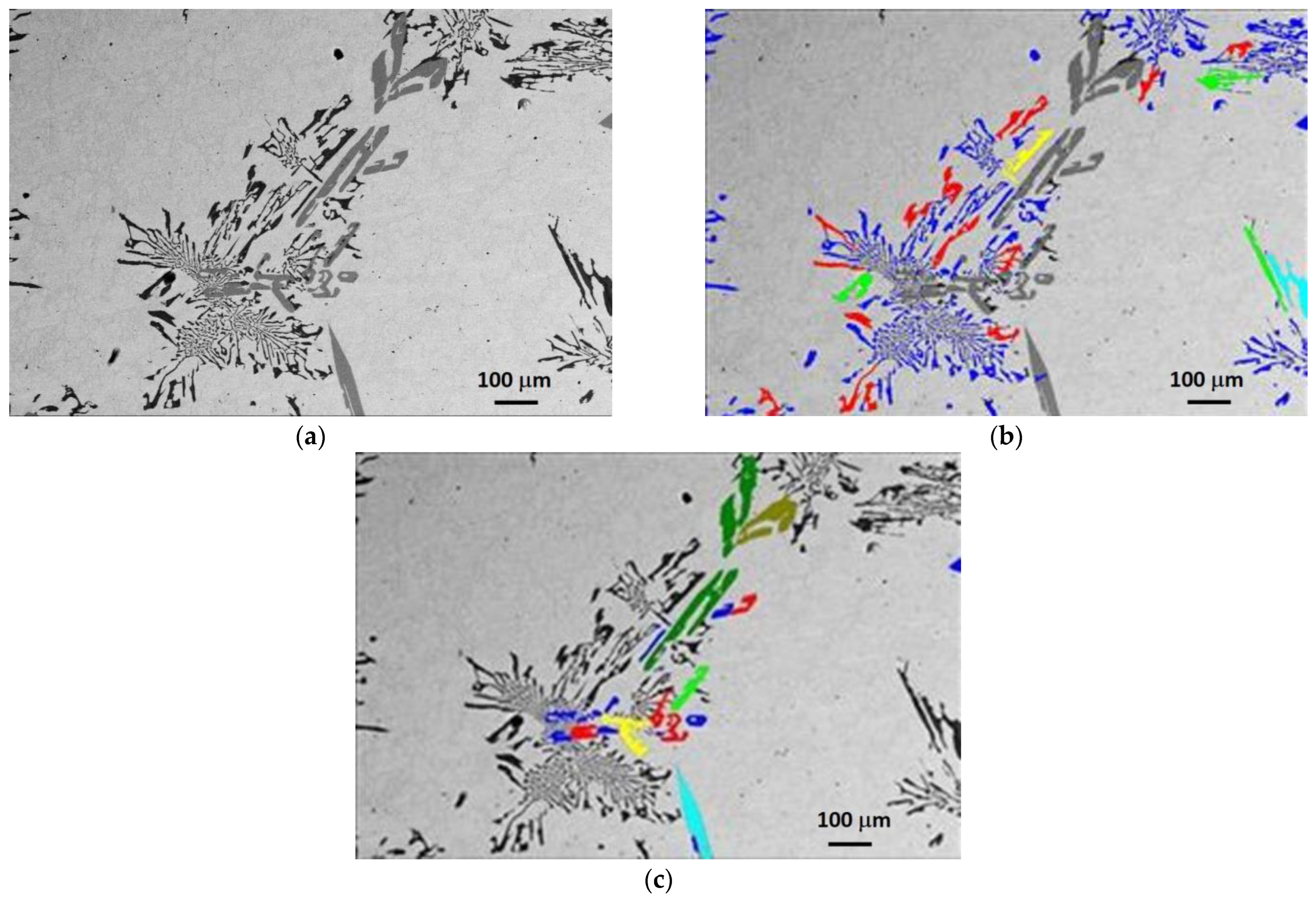
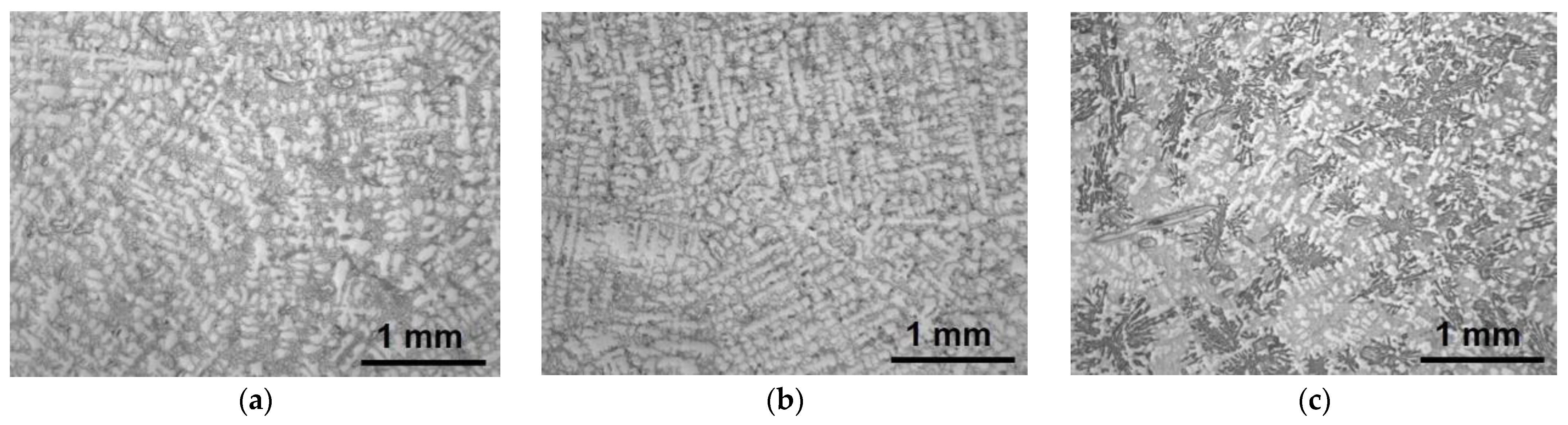
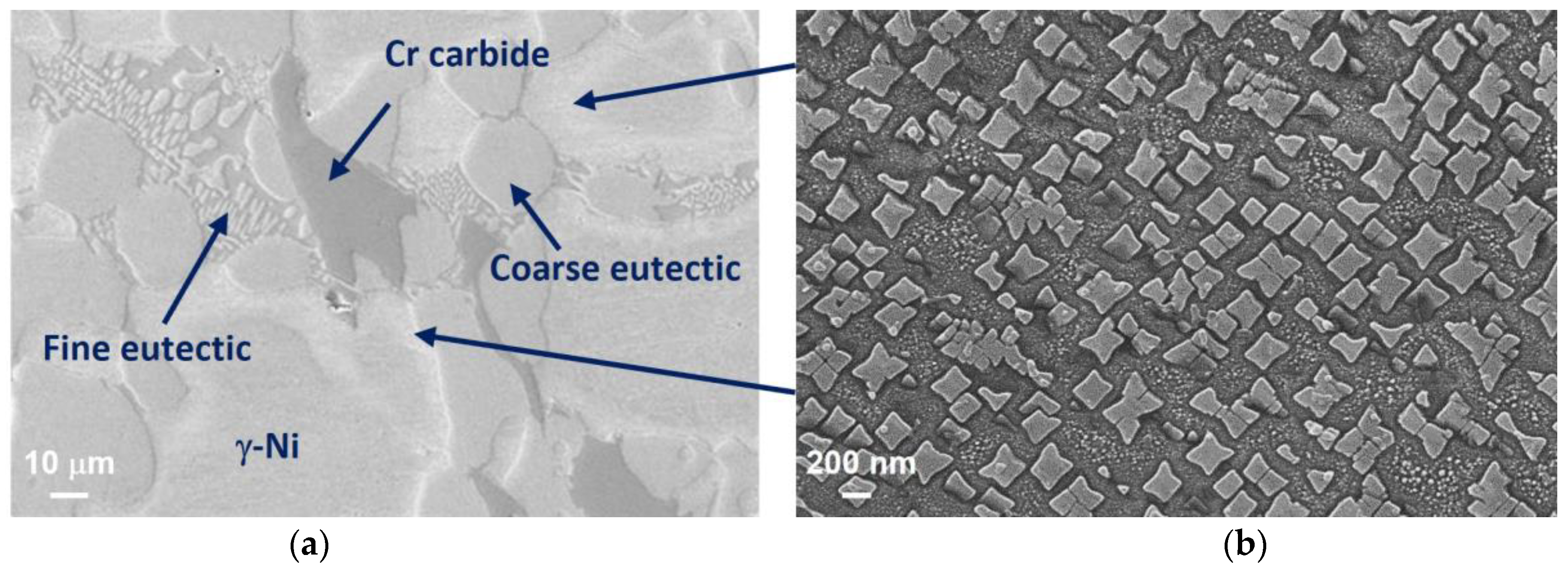
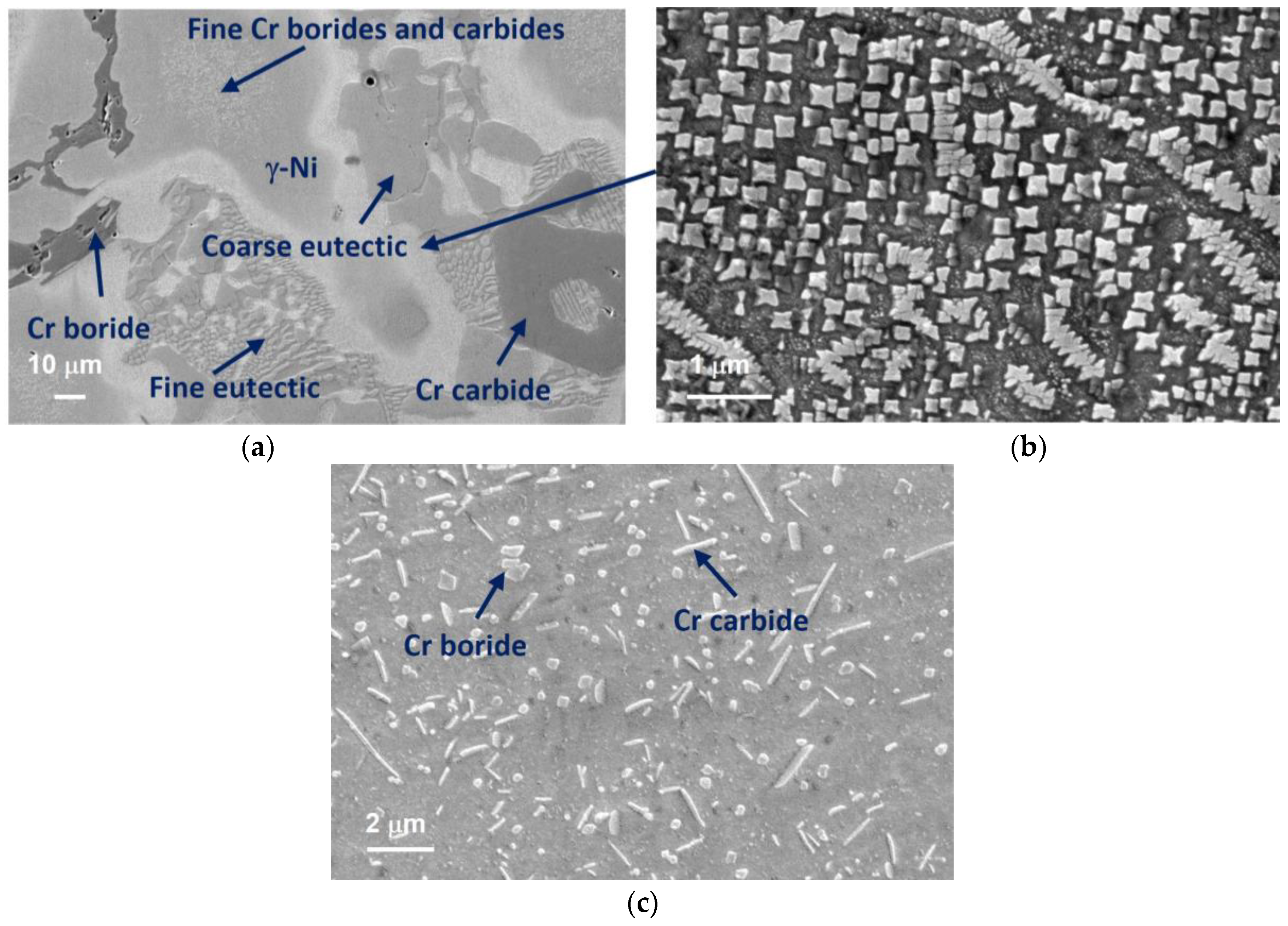
3.1. Microstructure Characterization Using Optical Microscopy
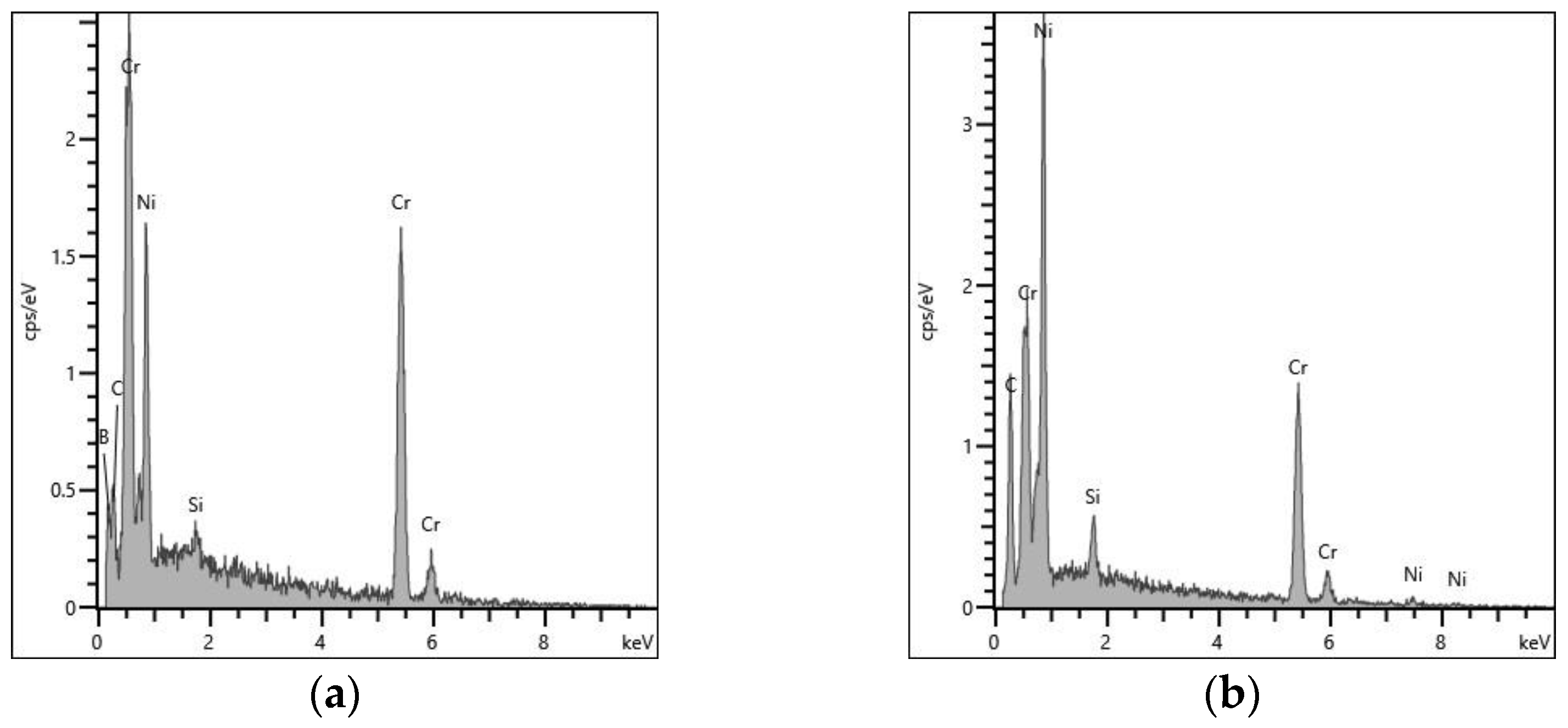
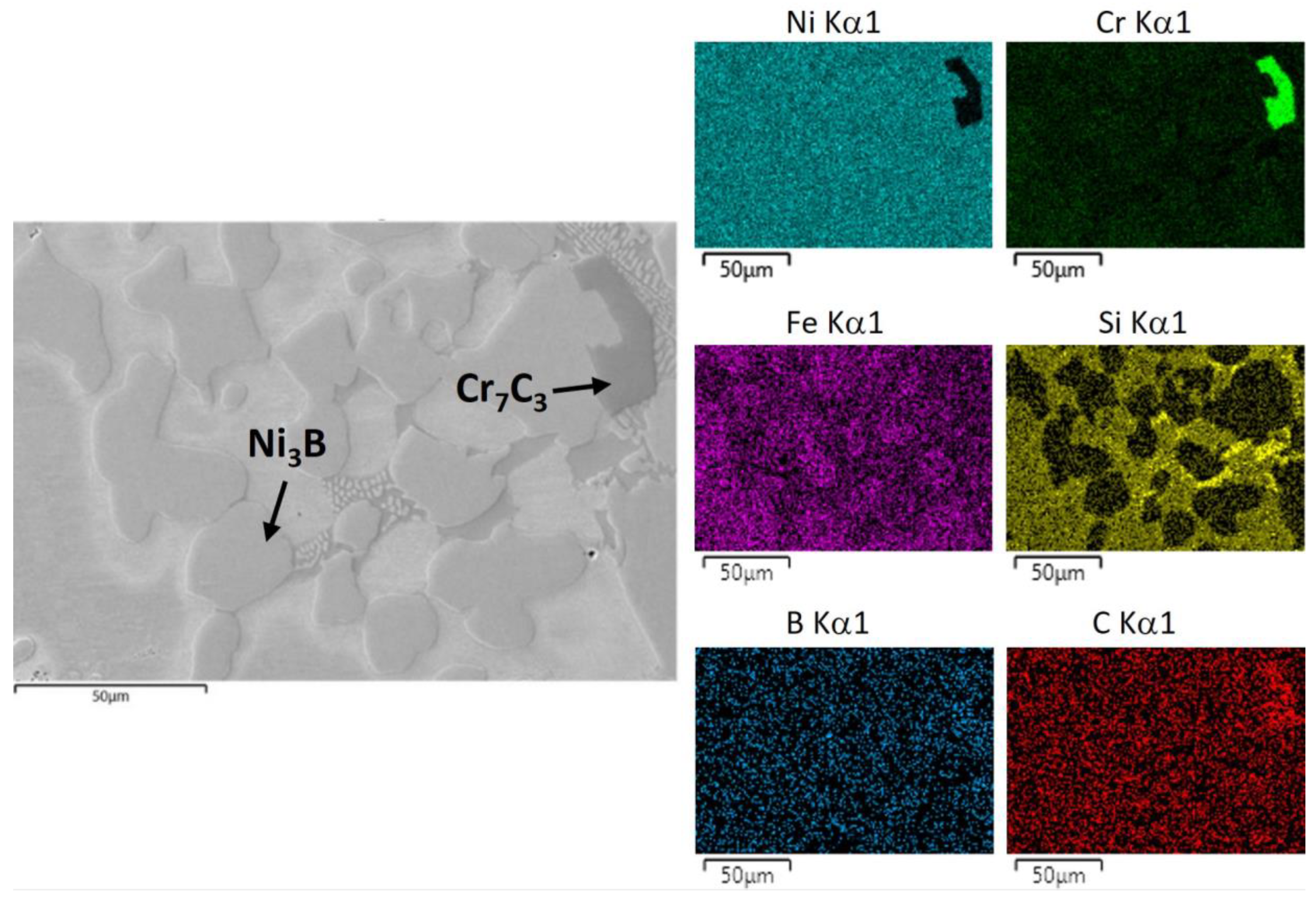
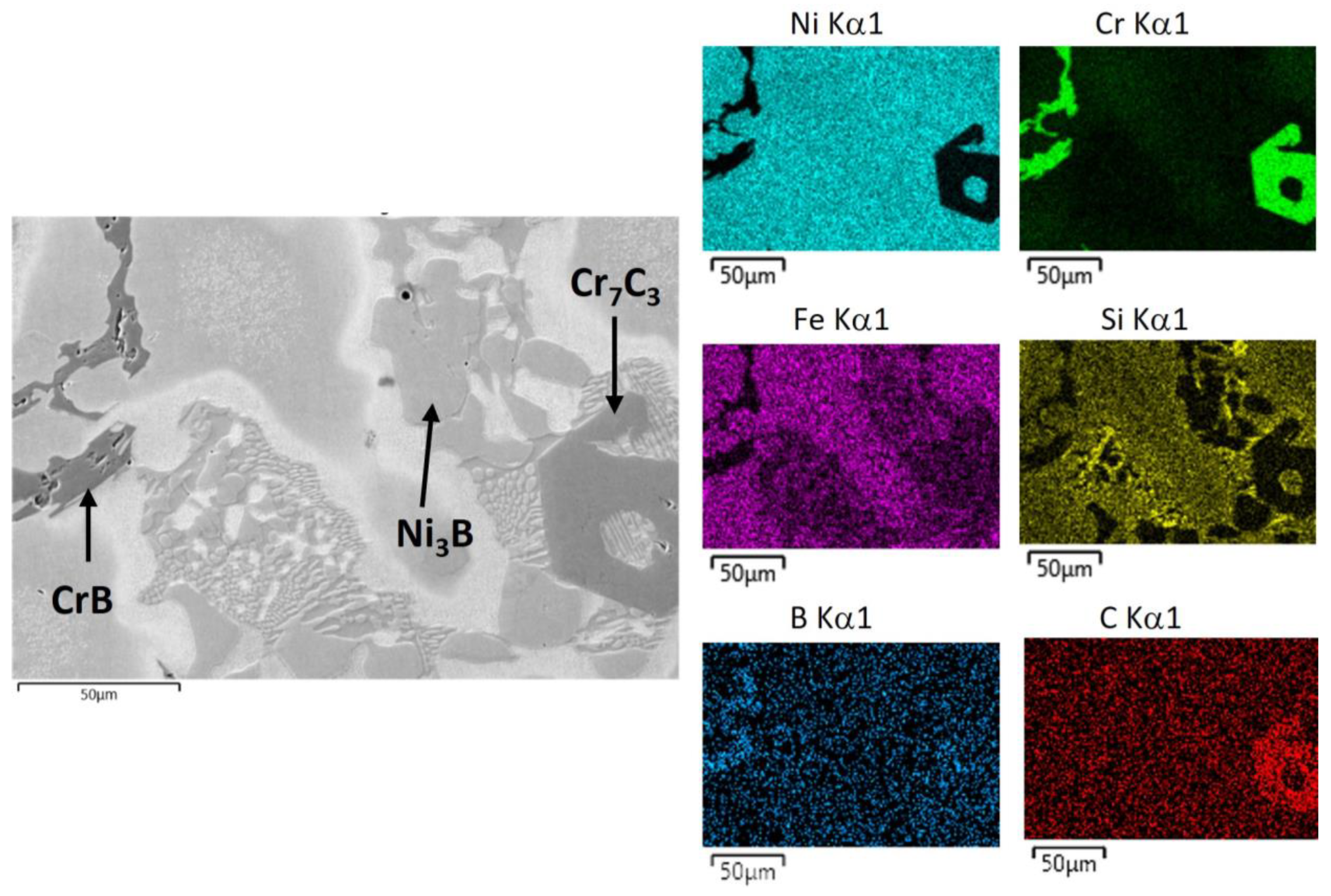
3.2. Phase Identification via FESEM/EDS/WDS/EBSD
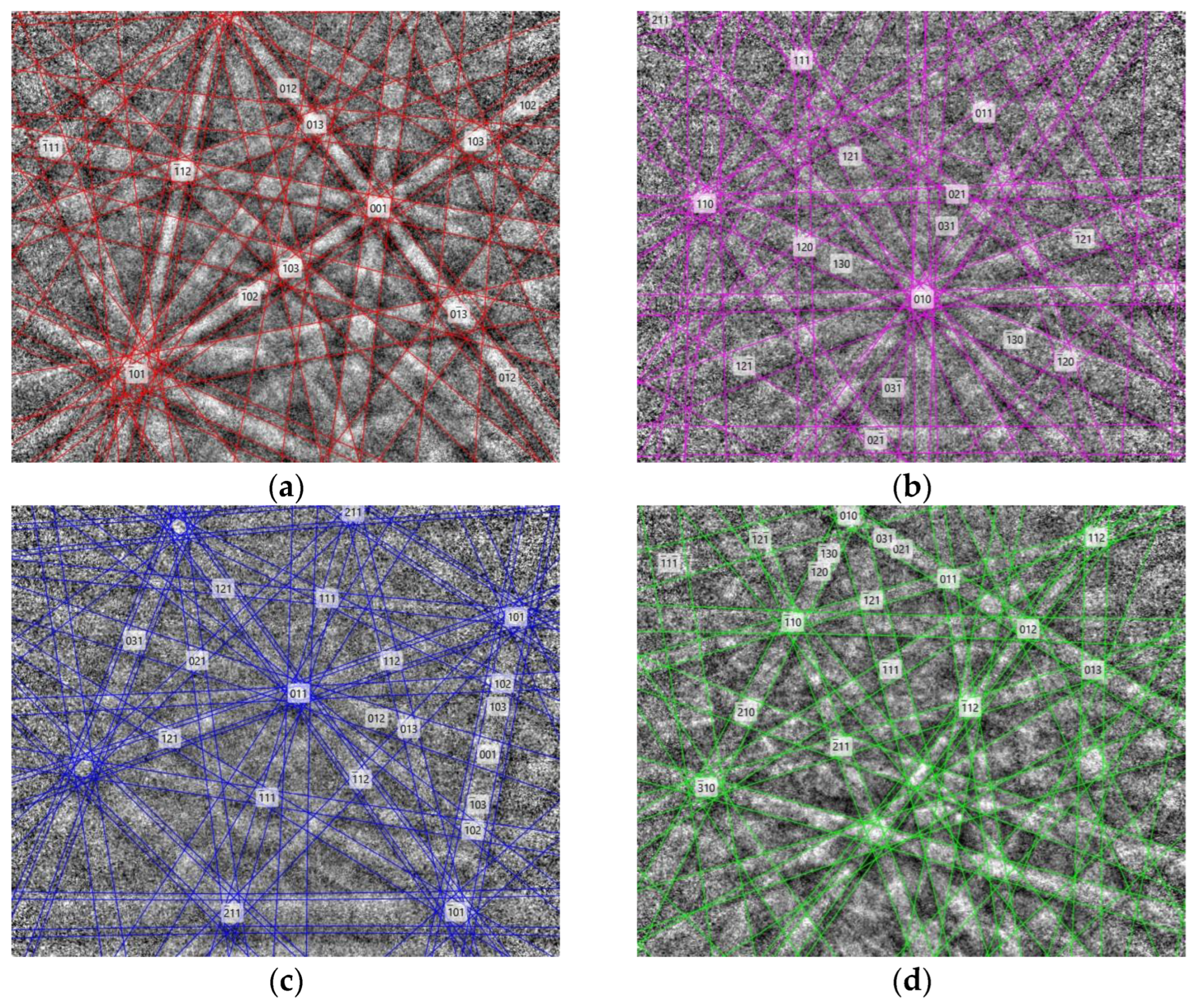
3.3. Identification of the Phases via X-ray Diffraction (XRD)
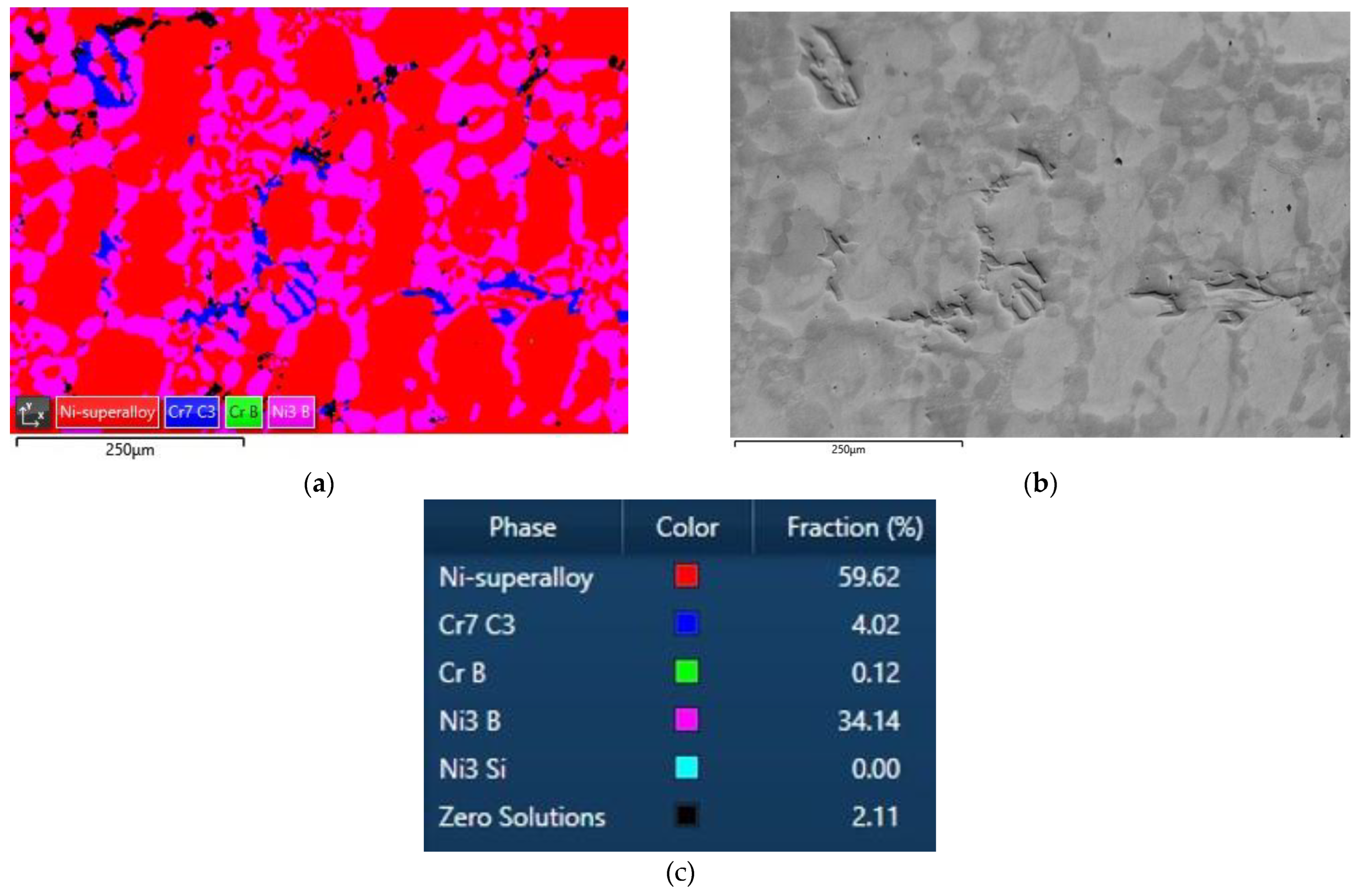
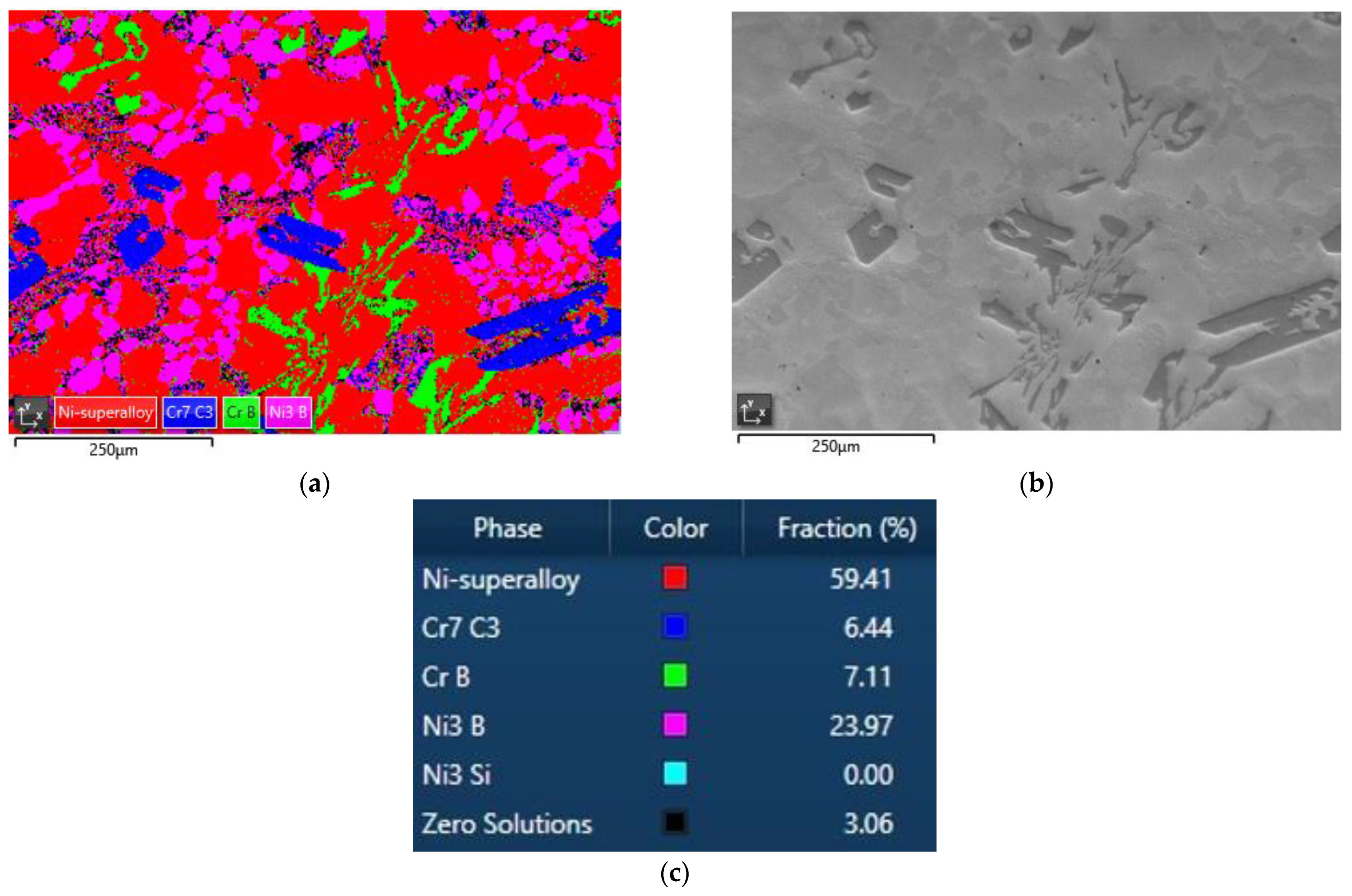
3.4. Quantification of Carbides and Borides in FESEM Images
3.5. Microindentation on the Main Phases Observed in the Microstructures
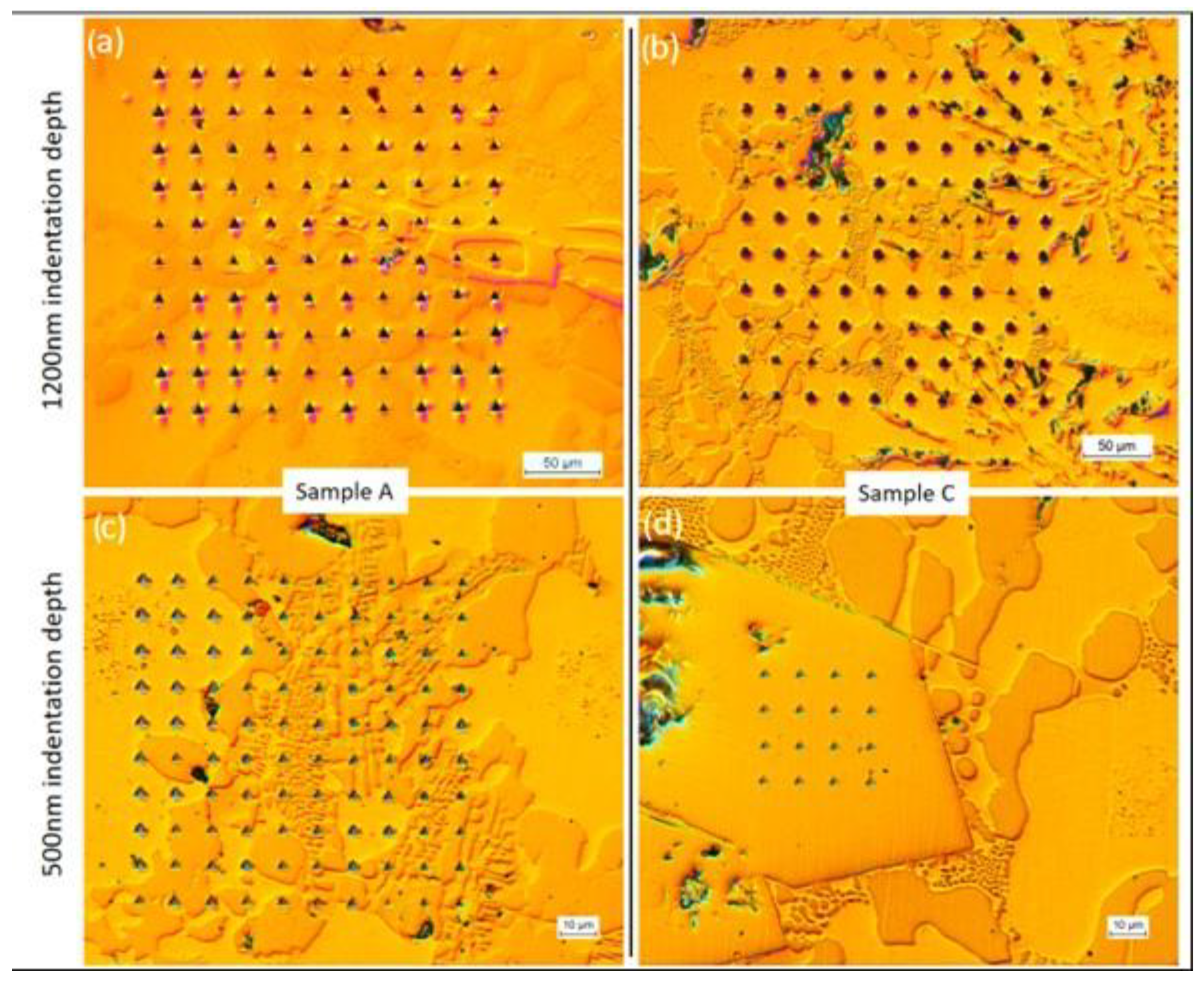
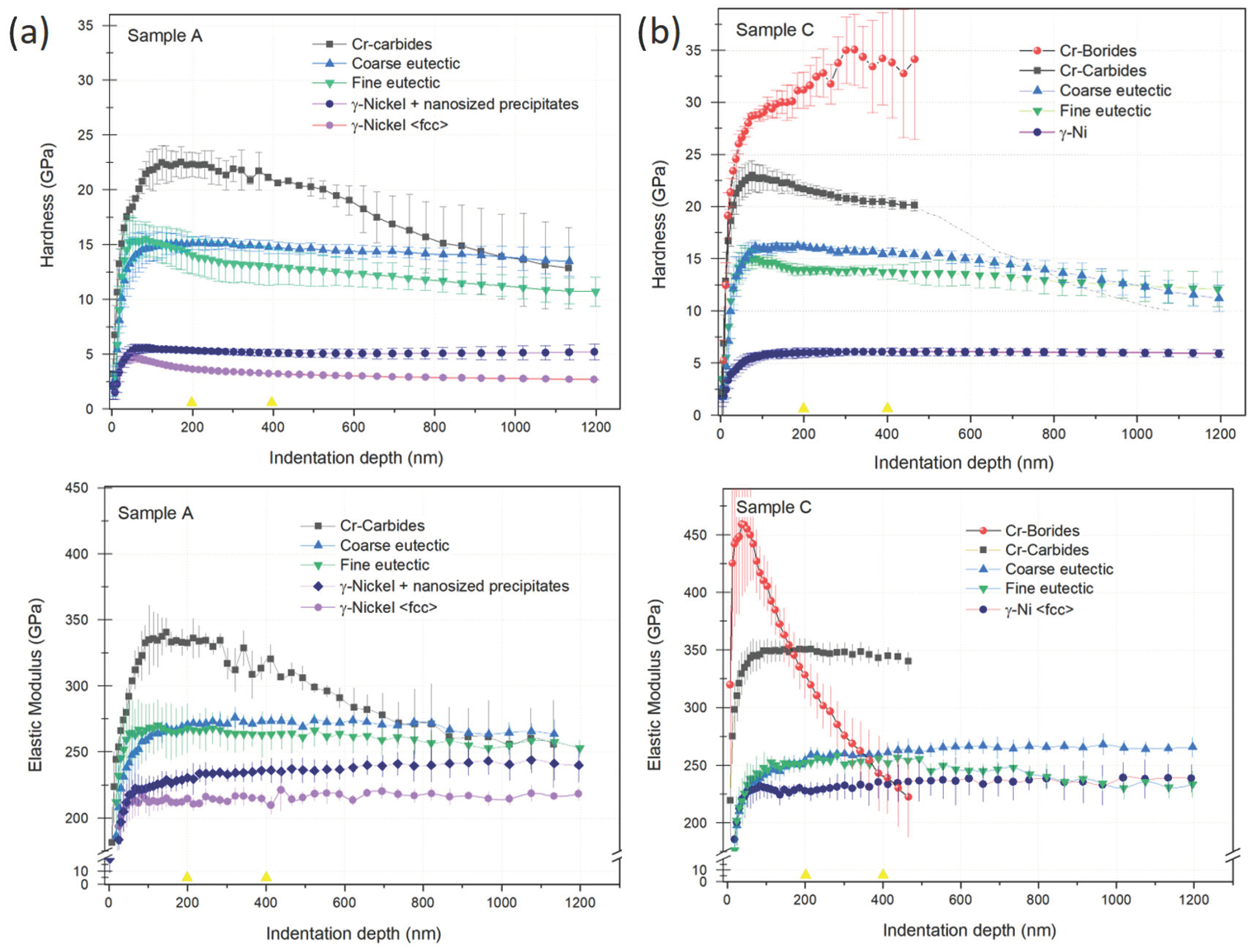
3.6. Nanoindentation on the Main Phases Observed in the Microstructures
3.7. Hot Hardness Tests
3.8. Equilibrium and Scheil Solidification Simulations
4. Conclusions
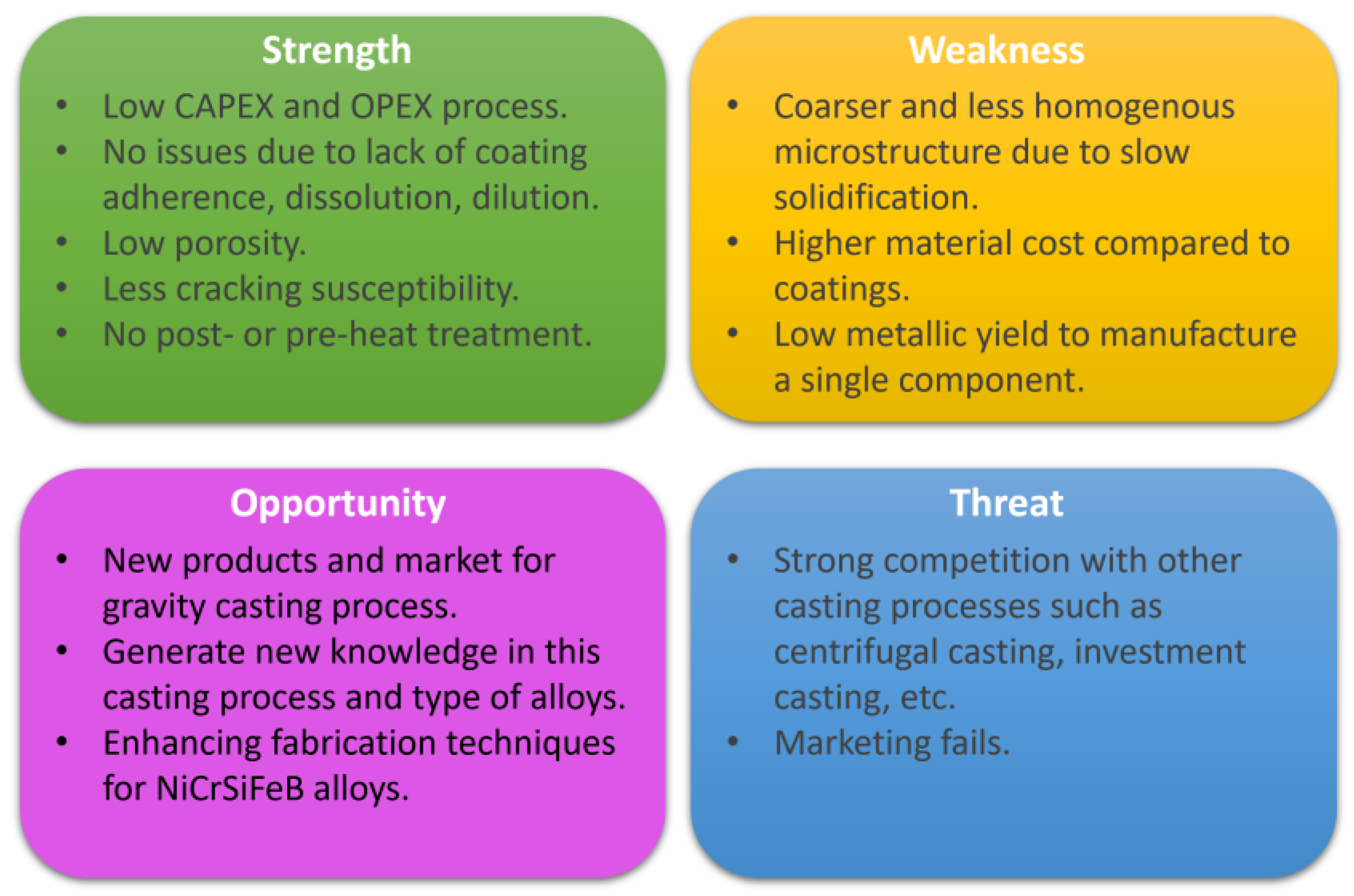
- Three NiCrFeSiFeB self-fluxing alloys were successfully manufactured using the gravity casting process with the aim of exploring their potential as an alternative to the materials that are produced employing conventional hardfacing and deposition technologies, as well as to substitute cobalt-based alloys in aircraft components. The microstructure evolution and strengthening mechanism was also established and compared to the LMD manufacturing route.
- The microstructure of the gravity casting samples consisted of soft γ-nickel dendrites, hard Cr-rich carbides and borides, as well as hard Ni3B eutectic phases. These phases were also observed in samples manufactured using the LMD technology. However, in the gravity casting process, the Cr-rich borides and carbides had a different morphology, as they were coarser and less homogeneously distributed in the γ-nickel matrix.
- The hardness of the studied alloys was found to be strongly related to the chemical composition, which determines the resulting phases that were formed during solidification.
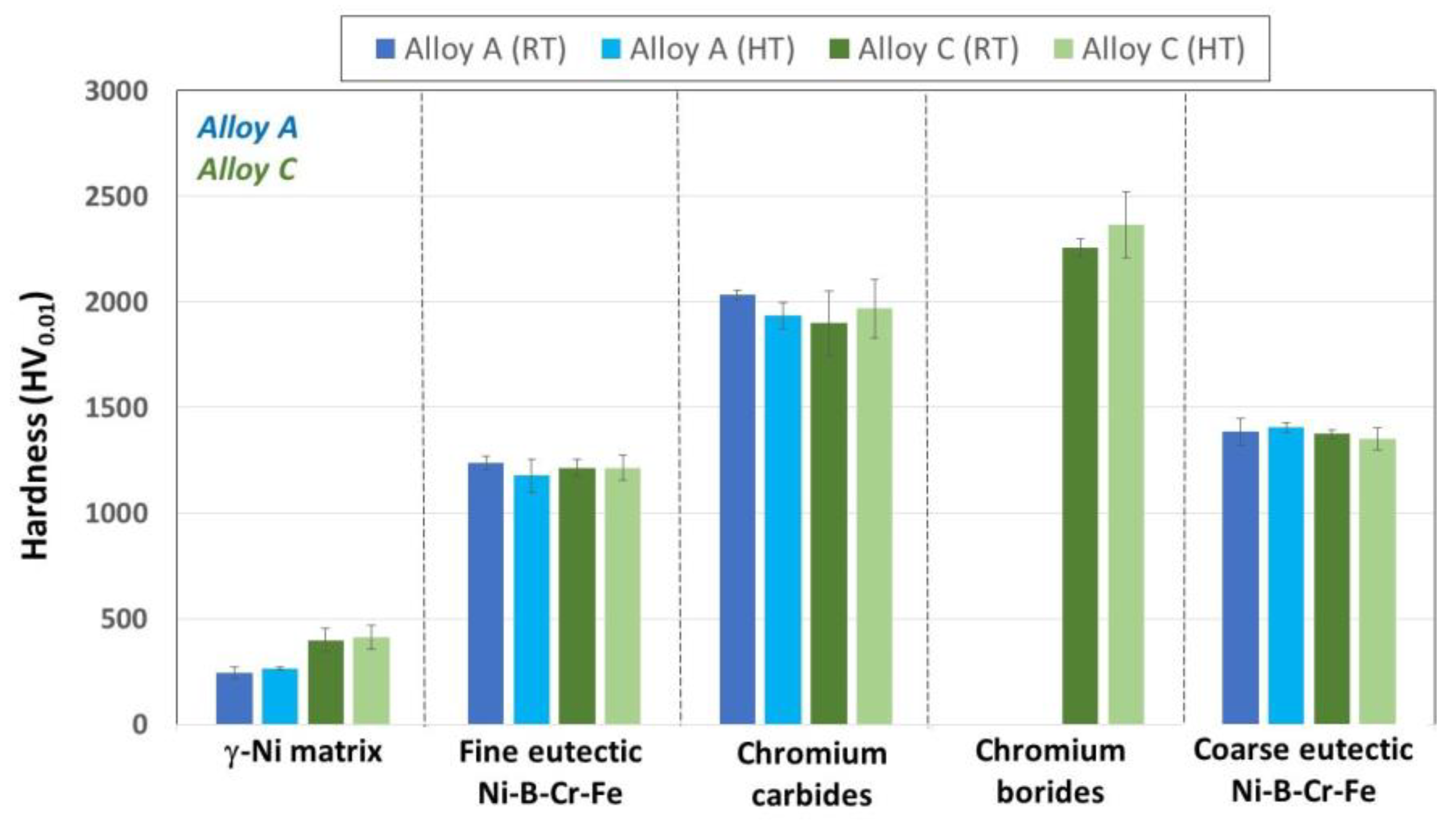
- The hardness of Alloy A, with its low content of C, B, and Cr, was mainly due to the hardness of the Ni3 (B, Fe, Cr) eutectic phase, with a small contribution from carbides.
- In Alloy C, with its high content of C, B, and Cr, the main strengthening mechanism was found to be the precipitation hardening of the especially hard carbides and borides that were randomly distributed in the γ-Ni matrix, as well as—to a lesser extent—by the hard Ni3(B, Fe, Cr) eutectic phase and the small contribution of the solid solution hardening of the γ-nickel dendrites. Additionally, the formation of the nanosized precipitates in the center and at the borders of the γ-nickel dendrites, were also observed as contributing to the strengthening of Alloy C. These precipitates were not formed in the samples of the same alloy manufactured through the LMD process.
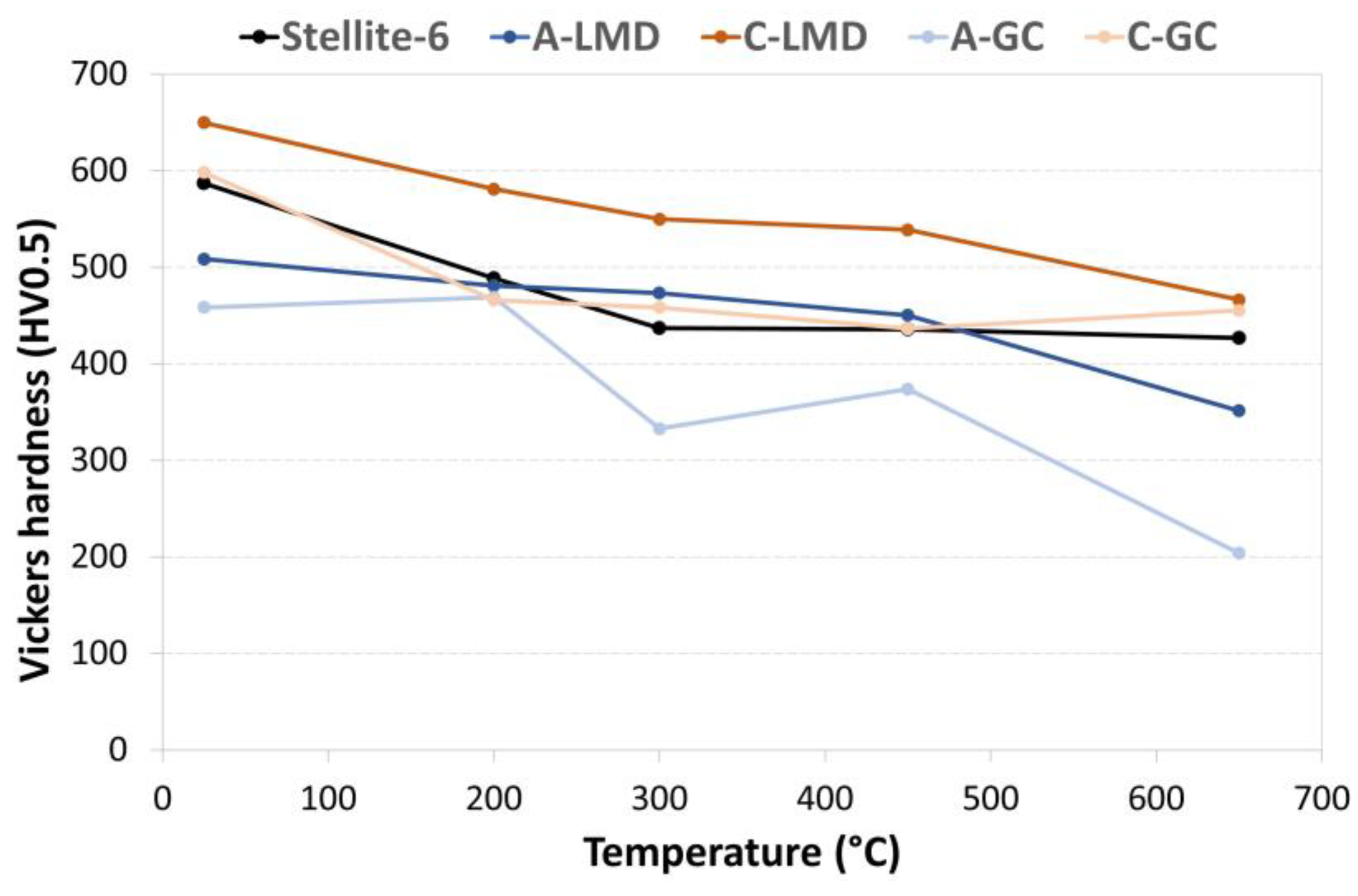
- The hot hardness (in the range of 25–650 °C) of Alloys A and C (which were manufactured via gravity casting) was lower than the one of samples that were manufactured via LMD. This could be due to the coarser and less homogenously distributed Cr-rich carbides and borides that are present in the gravity casting samples.
- However, the high alloy grade C, which was manufactured via gravity casting, showed a similar hot hardness to Stellite 6; thus, it could be a suitable candidate for the substitution of cobalt-based alloys in aircraft components.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simunovic, K.; Saric, T.; Simunovic, G. Different Approaches to the Investigation and Testing of the Ni-Based Self-Fluxing Alloy Coatings—A Review. Part 1: General Facts, Wear and Corrosion Investigations. Tribol. Trans. 2014, 57, 955–979. [Google Scholar] [CrossRef]
- Balaguru, S.; Gupta, M. Hardfacing Studies of Ni Alloys: A Critical Review. J. Mater. Res. Technol. 2021, 10, 1210–1242. [Google Scholar] [CrossRef]
- Kazamer, N.; Muntean, R.; Valean, P.C.; Pascal, D.T.; Marginean, G.; Serban, V.-A. Comparison of Ni-Based Self-Fluxing Remelted Coatings for Wear and Corrosion Applications. Materials 2021, 14, 3293. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.; Méndez, K.O.; Montoya, M.; Baltazar, V.H. Characterization of Flame Sprayed NiCrBSiMo Coatings Deposited with Different Spraying Parameters. Rev. De Metal. 2020, 56, e169. [Google Scholar] [CrossRef]
- Rachidi, R.; El Kihel, B.; Delaunois, F. Microstructure and Mechanical Characterization of NiCrBSi Alloy and NiCrBSi-WC Composite Coatings Produced by Flame Spraying. Mater. Sci. Eng. B 2019, 241, 13–21. [Google Scholar] [CrossRef]
- Navas, C.; Colaço, R.; de Damborenea, J.; Vilar, R. Abrasive Wear Behaviour of Laser Clad and Flame Sprayed-Melted NiCrBSi Coatings. Surf. Coat. Technol. 2006, 200, 6854–6862. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Luo, X.-T.; Zhang, S.-L.; Li, C.-J. A Novel Strategy for Depositing Dense Self-fluxing Alloy Coatings with Sufficiently Bonded Splats by One-Step Atmospheric Plasma Spraying. J. Therm. Spray Technol. 2020, 29, 173–184. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, J.; Sun, K.; Yang, H.; Cai, W.; Liu, Y.; Zhou, P.; Wu, S.; Li, H. Microstructural Charactistics of Plasma Sprayed NiCrBSi Coatings and Their Wear and Corrosion Behaviors. Coatings 2021, 11, 170. [Google Scholar] [CrossRef]
- Mrdak, M.; Vencl, A.L.; Ćosić, M. Microstructure and Mechanical Properties of the Mo-NiCrBSi Coating Deposited by Atmospheric Plasma spraying. FME Trans. 2009, 37, 27–32. [Google Scholar] [CrossRef]
- Krivorogova, A.S.; Ilinykh, N.I.; Ilinykh, S.A.; Gelchinskii, B.R. Theoretical and Experimental Study of Nickel-Based Self-Fluxing Materials. Russ. Metall. 2020, 8, 853–858. [Google Scholar] [CrossRef]
- Aoudia, K.; Retraint, D.; Verdy, C.; Langlade, C.; Creus, J.; Sanchette, F. Enhancement of Mechanical Properties and Corrosion Resistance of HVOF-Sprayed NiCrBSi Coatings Through Mechanical Attrition Treatment (SMAT). J. Therm. Spray Technol. 2020, 29, 2065–2079. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, M.; Upadhyaya, R. Wear and Friction Behavior of NiCrBSi Coatings at Elevated Temperatures. J. Therm. Spray Technol. 2019, 28, 1081–1102. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelík, V.; Csach, K.L.; de Hosson, J.T.M. Microstructure and Phase Formation in a Rapidly Solidified Laser-Deposited Ni-Cr-B-Si-C Hardfacing Alloy. Metall. Mater. Trans. A 2014, 45A, 878–892. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, J.; Song, R.; Bai, L.L.; Shao, J.Z.; Qu, C.C. Effect of the Scanning Speed on Microstructural Evolution and Wear Behaviors of Laser Cladding NiCrBSi Composite Coatings. Opt. Laser Technol. 2015, 72, 86–99. [Google Scholar] [CrossRef]
- Cheng, J.; Xing, Y.; Dong, E.; Zhao, L.; Liu, H.; Chang, T.; Chen, M.; Wang, J.; Lu, J.; Wan, J. An Overview of Laser Metal Deposition for Cladding: Defect Formation Mechanisms, Defect Suppression Methods and Performance Improvements of Laser-Cladded Layers. Materials 2022, 15, 5522. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Chen, J.; Zhao, J.; Yua, Y.; Zhou, H. High Temperature Wear Resistance of Laser Cladding NiCrBSi and NiCrBSi/WC-Ni Composite Coatings. Wear 2011, 270, 492–498. [Google Scholar] [CrossRef]
- Conde, A.; Zubiri, F.; de Damborenea, J. Cladding of Ni–Cr–B–Si Coatings with a High Power Diode Laser. J. Mater. Sci. Eng. A 2002, 334, 233–238. [Google Scholar] [CrossRef]
- Pereira, J.C.; Taboada, M.C.; Niklas, A.; Rayón, E.; Rocchi, J. Influence of the Chemical Composition on the Solidification Path, Strengthening Mechanisms and Hardness of Ni-Cr-Si-Fe-B Self-Fluxing Alloys Obtained by Laser-Directed Energy Deposition. Manuf. Mater. Process. 2023, 7, 110. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Zhang, X.; Shi, Z.; Tian, Y.; Lin, J. Microstructures and Mechanical Properties of Ni60 Alloy Fabricated by Laser Metal Deposition. Mater. Res. Express 2020, 7, 016569. [Google Scholar] [CrossRef]
- Zimogliadova, T.-A.; Bataev, A.A.; Lazurenko, D.V.; Bataev, I.A.; Bataev, V.A.; Golkovskii, M.G.; Saage, H.; Ogneva, T.S.; Ruktuev, A.A. Structural Characterization of Layers Fabricated by Non-Vacuum Electron Beam Cladding of Ni-Cr-Si-B Self-Fluxing Alloy with Additions of Niobium and Boron. Mater. Today Commun. 2022, 33, 104363. [Google Scholar] [CrossRef]
- Zimoglyadova, T.A.; Saage, H.; Pasichnik, V.A.; Egorova, A.S.; Matts, O. Structure and properties of functional Sel-Fluxing Nickel Containing Coatings Obtained by Non-Vacuum Electron Beam Cladding. Met. Sci. Heat Treat. 2019, 60, 633–640. [Google Scholar] [CrossRef]
- Shishkovskya, I.; Kakovkina, N.; Sherbakof, V. Mechanical Properties of NiCrBSi Self-Fluxing Alloy after LPBF with Additional Heating. Procedia CIRP 2020, 94, 217–221. [Google Scholar] [CrossRef]
- Deenadayalan, K.; Murali, V.; Elayaperumal, A.; Arulvel, S. Effective Role of Short Time Furnace Heat Treatment and Laser Treatment on the Residual Stress and Mechanical Properties of NiCrBSieWC Weldments Produced using Plasma Transferred Arc Welding Process. J. Mater. Res. Technol. 2021, 15, 3492–3513. [Google Scholar] [CrossRef]
- Simunovic, K.; Saric, T.; Simunovic, G. Different Approaches to the Investigation and Testing of the Ni-Based Self-Fluxing Alloy Coatings—A Review. Part 2: Microstructure, Adhesive Strength, Cracking Behavior, and Residual Stresses Investigations. Tribol. Trans. 2014, 57, 980–1000. [Google Scholar] [CrossRef]
- Deshpande, S.; Kulkarni, A.; Sampath, S.; Herman, H. Application of Image Analysis for Characterization of Porosity in Thermal Spray Coatings and Correlation with Small Angle Neutron Scattering. Surf. Coat. Technol. 2004, 187, 6–16. [Google Scholar] [CrossRef]
- Giacomantonio, M.; Gulizia, S.; Jahedi, M.; Wong, Y.; Moore, R.; Valimberti, M. Heat Treatment of the Thermally Sprayed Ni-Based Wear and Corrosion Coatings. Mater. Forum 2011, 35, 48–55. [Google Scholar]
- Chun, E.-J.; Park, C.; Nishikawa, H.; Kim, M.-S. Microstructural Characterization of Ni-based Self-Fluxing Alloy after Selective Surface-Engineering using Diode Laser. Appl. Surf. Sci. 2018, 442, 726–735. [Google Scholar] [CrossRef]
- Škamat, J.; Černašėjus, O.; Zhetessova, G.; Nikonova, T.; Zharkevich, O.; Višniakov, N. Effect of Laser Processing Parameters on Microstructure, Hardness and Tribology of NiCrCoFeCBSi/WC Coatings. Materials 2021, 14, 6034. [Google Scholar] [CrossRef]
- Afanaseva, L.E.; Ratkevich, G.V.; Novoselova, M.V. Role of Structural Factor in Elevation of Wear Resistance of a Ni-Cr-B-Si Coating after Laser Treatment. Met. Sci. Heat Treat. 2020, 61, 581–587. [Google Scholar] [CrossRef]
- Muntean, R.; Valean, P.-C.; Kazamer, N.; Utu, I.-D.; Marginean, G.; Serban, V.A. Effect of Feedstock Powder Intrinsic Characteristics on the Tribological Behavior of Inductively Remelted NiCrBSi Flame-Sprayed Coatings. Lubricants 2023, 11, 363. [Google Scholar] [CrossRef]
- Simunovic, K.; Havrlisan, S.; Saric, T.; Vukelic, D. Modeling and Optimization in Investigating Thermally Sprayed Ni-Based Self-Fluxing Alloy Coatings: A Review. Materials 2020, 13, 4584. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelík, V.; De Hosson, J.T.M. Dilution effects in laser cladding of Ni–Cr–B–Si–C hardfacing alloys. Mater. Lett. 2012, 84, 69–72. [Google Scholar] [CrossRef]
- Cuculea, D.C.; Ardelean, G.L.; Stanciu, E.M.; Roata, I.C.; Pascu, A. Dilution in Laser Cladding with Ni-Based Powders. Weld. Equip. Technol. 2021, 32, 56–60. [Google Scholar] [CrossRef]
- Da Silva, L.J.; D’Oliveira, A.S.C.M. NiCrSiBC coatings: Effect of dilution on microstructure and high temperature tribological behavior. Wear 2016, 350–351, 130–140. [Google Scholar] [CrossRef]
- Wanare, S.; Kalyankar, V. Influence of Fe Dilution and W Dissolution on Abrasive Wear Resistance of NICRBSI-WC Composite Hardfacing Deposited by Plasma Transferred Arc Hardfacing. J. Adv. Manuf. Syst. 2021, 21, 695–710. [Google Scholar] [CrossRef]
- Sadhu, A.; Choudhary, A.; Sarkar, S.; Nair, A.M.; Nayak, P.; Pawar, S.D.; Muvvala, G.; Pal, S.K.; Nath, A.K. A study on the Influence of Substrate Pre-Heating on Mitigation of Cracks in Direct Metal Laser Deposition of NiCrSiBC-60%WC Ceramic Coating on Inconel 718. Surf. Coat. Technol. 2020, 389, 125646. [Google Scholar] [CrossRef]
- Dai, Q.-L.; Luo, C.-B.; You, F.-Y. Crack Restraining Methods and their Effects on the Microstructures and Properties of Laser Cladded WC/Fe Coatings. Materials 2018, 11, 2541. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelík, V.; De Hosson, J.T.M. Advances in Laser Surface Engineering: Tackling the Cracking Problem in Laser Deposited Ni-Cr-B-Si-C Alloys. JOM 2013, 65, 741–748. [Google Scholar] [CrossRef]
- Hemmati, I.; Rao, J.C.; Ocelík, V.; De Hosson, J.T.M. Phase Formation and Properties of Vanadium-Modified Ni–Cr–B-Si–C Laser-Deposited Coatings. J. Mater. Sci. 2013, 48, 3315–3326. [Google Scholar] [CrossRef]
- Hemmati, I.; Ocelík, V.; de Hosson, J.T.M. Effects of the alloy composition on phase constitution and properties of laser deposited Ni-Cr-B-Si coatings. Phys. Procedia 2013, 41, 302–311. [Google Scholar] [CrossRef]
- Hemmati, I.; Rao, J.C.; Ocelik, V.; De Hosson, J.T.M. Electron Microscopy Characterization of Ni-Cr-B-Si-C Laser Deposited Coatings. Microsc. Microanal. 2013, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gómez-del Río, T.; Garrido, M.A.; Fernández, J.E.; Cadenas, M.; Rodríguez, J. Influence of the deposition techniques on the mechanical properties and microstructure of NiCrBSi coatings. J. Mater. Process. Technol. 2008, 204, 304–312. [Google Scholar] [CrossRef]
- Otsubo, F.; Era, H.; Kishitake, K. Structure and phases in nickel-base self-fluxing alloy coating containing high chromium and boron. J. Therm. Spray Technol. 2000, 9, 107–113. [Google Scholar] [CrossRef]







| Alloy | Chemical Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Ni | Cr | Si | Fe | B | C | Si/B | |
| A | 84.30 | 7.53 | 3.12 | 2.86 | 1.99 | 0.22 | 1.57 |
| B | 83.50 | 8.08 | 3.43 | 2.85 | 1.62 | 0.39 | 2.03 |
| C | 75.40 | 12.60 | 3.98 | 4.78 | 2.59 | 0.40 | 1.54 |
| γ-Ni Matrix | Boride | Carbide | Coarse Eutectic | Fine Eutectic | |||
|---|---|---|---|---|---|---|---|
| Ni-B-Cr-Fe | γ-Ni | Ni-B-Cr-Fe | Ni-Si-Fe-Cr | ||||
| Alloy A | |||||||
| B | - | 18.05 | * | - | * | - | |
| C | - | - | 20.84 | - | - | - | - |
| Si | 3.49 | - | - | 5.81 | - | 19.41 | |
| Cr | 7.88 | 79.63 | 77.84 | 6.66 | 9.02 | 5.17 | 0.86 |
| Fe | 3.72 | 0.54 | 0.37 | 2.10 | 4.17 | 2.04 | 0.63 |
| Ni | 84.91 | 1.78 | 1.20 | 91.24 | 80.99 | 92.80 | 79.32 |
| Alloy C | |||||||
| B | - | 48.465 | * | - | * | - | |
| C | - | - | 38.36 | - | - | - | - |
| Si | 10.58 | 0.30 | - | 7.79 | - | 26.84 | |
| Cr | 7.77 | 50.79 | 55.48 | 4.90 | 5.92 | 4.86 | 0.85 |
| Fe | 6.53 | - | 2.08 | 3.96 | 6.59 | 3.87 | 1.13 |
| Ni | 75.13 | 0.44 | 4.08 | 90.98 | 79.69 | 90.97 | 71.18 |
| Phase/Precipitate | Sample A | Sample C | ||
|---|---|---|---|---|
| 25 °C | 650 °C | 25 °C | 650 °C | |
| γ-Ni fcc | 244 ± 26 | 265 ± 7 | 398 ± 54 | 412 ± 55 |
| Fine eutectic | 1238 ± 32 | 1178 ± 79 | 1215 ± 41 | 1214 ± 60 |
| Coarse eutectic | 1385 ± 64 | 1406 ± 23 | 1374 ± 19 | 1352 ± 53 |
| Cr-Carbides | 2033 ± 23 | 1935 ± 63 | 1899 ± 153 | 1968 ± 138 |
| Cr-Borides | --- | --- | 2257 ± 44 | 2366 ± 157 |
| Phase/Precipitate | Sample A | Sample C | ||
|---|---|---|---|---|
| Hardness (GPa) | Elastic Modulus (GPa) | Hardness (GPa) | Elastic Modulus (GPa) | |
| γ-Ni fcc | 3.4 ± 0.1 | 216 ± 2.5 | 5.9 ± 0.1 | 260 ± 2.0 |
| γ-Ni with the presence of nano precipitates near the dendritic borders | 5.1± 0.1 | 235± 2.5 | --- | --- |
| Fine eutectics | 12 ± 1.4 | 266 ± 1.3 | 13.6 ± 1.3 | 270 ± 1.0 |
| Coarse eutectics | 16 ± 0.1 | 273 ± 2.1 | 16 ± 0.2 | 272 ± 3.2 |
| Cr carbides | 23 ± 0.1 | 329 ± 1.3 | 25 ± 0.3 | 339 ± 2.5 |
| Cr borides | --- | 35.5 ± 4.5 | >400 | |
| Alloy | T liq. (°C) | T sol. (°C) | ΔT (°C) |
|---|---|---|---|
| A | 1244.3 | 968.5 | 275.8 |
| C | 1299.9 | 955.6 | 344.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niklas, A.; Santos, F.; Garcia, D.; Rouco, M.; González-Martínez, R.; Pereira, J.C.; Rayón, E.; Lopez, P.; Guillonneau, G. Chemical Composition Effects on the Microstructure and Hot Hardness of NiCrSiFeB Self-Fluxing Alloys Manufactured via Gravity Casting. J. Manuf. Mater. Process. 2023, 7, 196. https://doi.org/10.3390/jmmp7060196
Niklas A, Santos F, Garcia D, Rouco M, González-Martínez R, Pereira JC, Rayón E, Lopez P, Guillonneau G. Chemical Composition Effects on the Microstructure and Hot Hardness of NiCrSiFeB Self-Fluxing Alloys Manufactured via Gravity Casting. Journal of Manufacturing and Materials Processing. 2023; 7(6):196. https://doi.org/10.3390/jmmp7060196
Chicago/Turabian StyleNiklas, Andrea, Fernando Santos, David Garcia, Mikel Rouco, Rodolfo González-Martínez, Juan Carlos Pereira, Emilio Rayón, Patricia Lopez, and Gaylord Guillonneau. 2023. "Chemical Composition Effects on the Microstructure and Hot Hardness of NiCrSiFeB Self-Fluxing Alloys Manufactured via Gravity Casting" Journal of Manufacturing and Materials Processing 7, no. 6: 196. https://doi.org/10.3390/jmmp7060196






